Rifabutin-Containing Triple Therapy (RHB-105) for Eradication of Helicobacter pylori: Randomized ERADICATE Hp Trial
- PMID: 33050205
- PMCID: PMC7600817
- DOI: 10.3390/antibiotics9100685
Rifabutin-Containing Triple Therapy (RHB-105) for Eradication of Helicobacter pylori: Randomized ERADICATE Hp Trial
Abstract
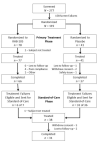
Due to increasing resistance to commonly used antibiotics, the World Health Organization and Food and Drug Administration have advocated the development of new therapeutic regimens for Helicobacter pylori (H. pylori). This phase three, double-blind study (ERADICATE Hp) randomized (2:1) treatment-naïve adults with H. pylori infection and dyspepsia to RHB-105 (an all-in-one combination of omeprazole 40 mg, amoxicillin 1000 mg, and rifabutin 50 mg) or an identically-appearing placebo, both administered every 8 h for 14 days. The H. pylori eradication rate with RHB-105, using a modified intent-to-treat (mITT) population of subjects who received ≥1 dose of study drug and had test-of-eradication performed 28-35 days post-completion of therapy, was compared (one-sample Z-test) to a literature-derived comparator rate of 70% and success rate with physician-selected standard-of-care given to placebo failures. The mITT H. pylori eradication rate (95% CI) with RHB-105 of 89.4% (82.0-96.8%) was greater than both the literature-derived comparator rate (P < 0.001) and the standard-of-care rate of 63.0% (44.8-81.1%) (P = 0.006). Adverse events with an incidence ≥5% for RHB-105 were diarrhea (12.7%), headache (11.9%), chromaturia (9.3%), abdominal tenderness (6.8%), and dizziness (5.1%). No leukopenia was noted. RHB-105 (Talicia®) proved to be a safe and effective empiric therapy for H. pylori eradication.
Keywords: Helicobacter pylori; RHB-105; clinical trial; dyspepsia; rifabutin; standard-of-care.
Conflict of interest statement
D.Y.G. is a consultant for RedHill Biopharma and Phathom Pharmaceuticals regarding novel
Figures
References
-
- Barzilay E.J., Fagan R.P. 2012 Yellow Book—Travelers’ Health—CDC. Oxford University Press Inc.; New York, NY, USA: 2011. Chapter 3: Infectious Diseases Related to Travel.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous