Neuropathic Itch
- PMID: 33050211
- PMCID: PMC7601786
- DOI: 10.3390/cells9102263
Neuropathic Itch
Abstract
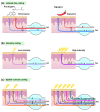
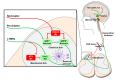
Neurologic insults as varied as inflammation, stroke, and fibromyalgia elicit neuropathic pain and itch. Noxious sensation results when aberrantly increased afferent signaling reaches percept-forming cortical neurons and can occur due to increased sensory signaling, decreased inhibitory signaling, or a combination of both processes. To treat these symptoms, detailed knowledge of sensory transmission, from innervated end organ to cortex, is required. Molecular, genetic, and behavioral dissection of itch in animals and patients has improved understanding of the receptors, cells, and circuits involved. In this review, we will discuss neuropathic itch with a focus on the itch-specific circuit.
Keywords: inflammation; neuropathy; pruritus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- International Association for the Study of Pain Home Page. [(accessed on 20 September 2020)]; Available online: https://www.iasp-pain.org/GlobalYear/NeuropathicPain.
-
- Oaklander A.L. Neuropathic Itch. In: Carstens E., Akiyama T., editors. Itch: Mechanisms and Treatment. 1st ed. CRC Press; Boca Raton, FL, USA: 2014.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

