Senescence-Associated Secretory Phenotype Determines Survival and Therapeutic Response in Cervical Cancer
- PMID: 33050319
- PMCID: PMC7601905
- DOI: 10.3390/cancers12102899
Senescence-Associated Secretory Phenotype Determines Survival and Therapeutic Response in Cervical Cancer
Abstract
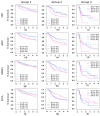
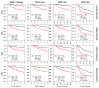
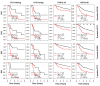
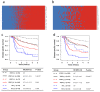
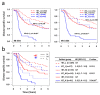
Molecular biomarkers that can predict survival and therapeutic outcome are still lacking for cervical cancer. Here we measured a panel of 19 serum proteins in sera from 565 patients with stage II or III cervical cancer and identified 10 proteins that have an impact on disease specific survival (DSS) (Hazzard's ratio; HR = 1.51-2.1). Surprisingly, all ten proteins are implicated in senescence-associated secreted phenotype (SASP), a hallmark of cellular senescence. Machine learning using Ridge regression of these SASP proteins can robustly stratify patients with high SASP, which is associated with poor survival, and patients with low SASP associated with good survival (HR = 3.09-4.52). Furthermore, brachytherapy, an effective therapy for cervical cancer, greatly improves survival in SASP-high patients (HR = 3.3, p < 5 × 10-5) but has little impact on survival of SASP-low patients (HR = 1.5, p = 0.31). These results demonstrate that cellular senescence is a major determining factor for survival and therapeutic response in cervical cancer and suggest that senescence reduction therapy may be an efficacious strategy to improve the therapeutic outcome of cervical cancer.
Keywords: biomarkers; cervical neoplasia; gynecologic cancers; prognosis; proteomics; radiation therapy; serum proteins.
Conflict of interest statement
The authors declare no conflict of interest. BDS is employed by Jinfiniti Precision Medicine LLC.
Figures
References
-
- Walboomers J.M., Jacobs M.V., Manos M.M., Bosch F.X., Kummer J.A., Shah K.V., Snijders P.J., Peto J., Meijer C.J., Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

