Therapeutic Implications for PDE2 and cGMP/cAMP Mediated Crosstalk in Cardiovascular Diseases
- PMID: 33050419
- PMCID: PMC7590001
- DOI: 10.3390/ijms21207462
Therapeutic Implications for PDE2 and cGMP/cAMP Mediated Crosstalk in Cardiovascular Diseases
Abstract
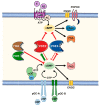
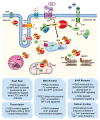
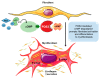
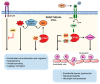
Phosphodiesterases (PDEs) are the principal superfamily of enzymes responsible for degrading the secondary messengers 3',5'-cyclic nucleotides cAMP and cGMP. Their refined subcellular localization and substrate specificity contribute to finely regulate cAMP/cGMP gradients in various cellular microdomains. Redistribution of multiple signal compartmentalization components is often perceived under pathological conditions. Thereby PDEs have long been pursued as therapeutic targets in diverse disease conditions including neurological, metabolic, cancer and autoimmune disorders in addition to numerous cardiovascular diseases (CVDs). PDE2 is a unique member of the broad family of PDEs. In addition to its capability to hydrolyze both cAMP and cGMP, PDE2 is the sole isoform that may be allosterically activated by cGMP increasing its cAMP hydrolyzing activity. Within the cardiovascular system, PDE2 serves as an integral regulator for the crosstalk between cAMP/cGMP pathways and thereby may couple chronically adverse augmented cAMP signaling with cardioprotective cGMP signaling. This review provides a comprehensive overview of PDE2 regulatory functions in multiple cellular components within the cardiovascular system and also within various subcellular microdomains. Implications for PDE2- mediated crosstalk mechanisms in diverse cardiovascular pathologies are discussed highlighting the prospective use of PDE2 as a potential therapeutic target in cardiovascular disorders.
Keywords: NO signaling; PDE2; arrhythmia; cAMP/cGMP crosstalk; cardiovascular disease; heart failure; inflammation; natriuretic peptides.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Kaptoge S., Pennells L., De Bacquer D., Cooney M.T., Kavousi M., Stevens G., Riley L.M., Savin S., Khan T., Altay S., et al. World Health Organization cardiovascular disease risk charts: Revised models to estimate risk in 21 global regions. Lancet Glob. Health. 2019;7:1332–1345. doi: 10.1016/S2214-109X(19)30318-3. - DOI - PMC - PubMed
-
- Roth G.A., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J., Abdelalim A., et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. - DOI - PMC - PubMed
-
- Nelson S., Whitsel L., Khavjou O., Phelps D., Leib A. Projections of Cardiovascular Disease Prevalence and Costs: 2015–2035. Technical Report for American Heart Association; Washington, DC, USA: 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

