Synergic Therapeutic Potential of PEA-Um Treatment and NAAA Enzyme Silencing In the Management of Neuroinflammation
- PMID: 33050589
- PMCID: PMC7589809
- DOI: 10.3390/ijms21207486
Synergic Therapeutic Potential of PEA-Um Treatment and NAAA Enzyme Silencing In the Management of Neuroinflammation
Abstract
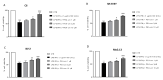
Inflammation is a key element in the pathobiology of neurodegenerative diseases and sees the involvement of different neuronal and non-neuronal cells as players able to respond to inflammatory signals of immune origin. Palmitoylethanolamide (PEA) is an endogenous potent anti-inflammatory agent, in which activity is regulated by N-acylethanolamine acid amidase (NAAA), that hydrolyzes saturated or monounsaturated fatty acid ethanolamides, such as PEA. In this research, an in vitro study was performed on different neuronal (SH-SY5Y) and non-neuronal cell lines (C6, BV-2, and Mo3.13) subjected to NAAA enzyme silencing and treated with PEA ultra-micronized (PEA-um) (1, 3, and 10 μM) to increase the amount of endogenous PEA available for counteract neuroinflammation provoked by stimulation with lipopolysaccharide (LPS) (1 μg/mL) and interferon gamma (INF-γ )(100 U/mL). Cell viability was performed by MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) staining, suggesting a protective effect of PEA-um (3 and 10 μM) on all cell lines studied. Western Blot analysis for inflammatory markers (Inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2)) was carried out in control and NAAA-silenced cells, highlighting how the concomitant treatment of the neuronal and non-neuronal cells with PEA-um after NAAA genic downregulation is satisfactory to counteract neuroinflammation. These in vitro findings support the protective role of endogenous PEA availability in the neuronal field, bringing interesting information for a translational point of view.
Keywords: glioma; microglia; neuroblastoma; neuroinflammation; oligodendrocytes; palmitoylethanolamide.
Conflict of interest statement
S.C. is co-inventor on patent WO2013121449 A8 (Epitech Group Srl) which deals with methods and compositions for the modulation of amidases capable of hydrolyzing N-acylethanolamines employable in the treatment of inflammatory diseases. This invention is wholly unrelated to the present study. Moreover, S.C. is also, with Epitech Group, a co-inventor on the following patents: EP 2 821 083; MI2014 A001495; 102015000067344 that are however unrelated to the study. This does not alter our adherence to journal policies on sharing data and materials. The remaining authors declare no conflict of interest.
Figures






References
-
- Ribeiro A., Pontis S., Mengatto L., Armirotti A., Chiurchiu V., Capurro V., Fiasella A., Nuzzi A., Romeo E., Moreno-Sanz G., et al. A Potent Systemically Active N-Acylethanolamine Acid Amidase Inhibitor that Suppresses Inflammation and Human Macrophage Activation. ACS Chem. Biol. 2015;10:1838–1846. doi: 10.1021/acschembio.5b00114. - DOI - PMC - PubMed
-
- Petrosino S., Campolo M., Impellizzeri D., Paterniti I., Allara M., Gugliandolo E., D’Amico R., Siracusa R., Cordaro M., Esposito E., et al. 2-Pentadecyl-2-Oxazoline, the Oxazoline of Pea, Modulates Carrageenan-Induced Acute Inflammation. Front. Pharmacol. 2017;8:308. doi: 10.3389/fphar.2017.00308. - DOI - PMC - PubMed
-
- Impellizzeri D., Bruschetta G., Cordaro M., Crupi R., Siracusa R., Esposito E., Cuzzocrea S. Micronized/ultramicronized palmitoylethanolamide displays superior oral efficacy compared to nonmicronized palmitoylethanolamide in a rat model of inflammatory pain. J. Neuroinflammation. 2014;11:136. doi: 10.1186/s12974-014-0136-0. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

