Biomedical Science to Tackle the COVID-19 Pandemic: Current Status and Future Perspectives
- PMID: 33050601
- PMCID: PMC7587204
- DOI: 10.3390/molecules25204620
Biomedical Science to Tackle the COVID-19 Pandemic: Current Status and Future Perspectives
Abstract
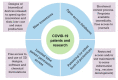
The coronavirus infectious disease (COVID-19) pandemic emerged at the end of 2019, and was caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), which has resulted in an unprecedented health and economic crisis worldwide. One key aspect, compared to other recent pandemics, is the level of urgency, which has started a race for finding adequate answers. Solutions for efficient prevention approaches, rapid, reliable, and high throughput diagnostics, monitoring, and safe therapies are needed. Research across the world has been directed to fight against COVID-19. Biomedical science has been presented as a possible area for combating the SARS-CoV-2 virus due to the unique challenges raised by the pandemic, as reported by epidemiologists, immunologists, and medical doctors, including COVID-19's survival, symptoms, protein surface composition, and infection mechanisms. While the current knowledge about the SARS-CoV-2 virus is still limited, various (old and new) biomedical approaches have been developed and tested. Here, we review the current status and future perspectives of biomedical science in the context of COVID-19, including nanotechnology, prevention through vaccine engineering, diagnostic, monitoring, and therapy. This review is aimed at discussing the current impact of biomedical science in healthcare for the management of COVID-19, as well as some challenges to be addressed.
Keywords: clinical trials; diagnostics; nanomedicine; prevention; treatment; vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Sivasankarapillai V.S., Pillai A.M., Rahdar A., Sobha A.P., Das S.S., Mitropoulos A.C., Mokarrar M.H., Kyzas G.Z. On Facing the SARS-CoV-2 (COVID-19) with Combination of Nanomaterials and Medicine: Possible Strategies and First Challenges. Nanomaterials. 2020;10:852. doi: 10.3390/nano10050852. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

