House Dust Mite Exposure Causes Increased Susceptibility of Nasal Epithelial Cells to Adenovirus Infection
- PMID: 33050625
- PMCID: PMC7600414
- DOI: 10.3390/v12101151
House Dust Mite Exposure Causes Increased Susceptibility of Nasal Epithelial Cells to Adenovirus Infection
Abstract
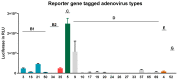
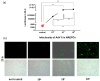
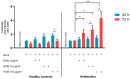
Adenovirus (AdV) infections in the respiratory tract may cause asthma exacerbation and allergic predisposition, and the house dust mite (HDM) may aggravate virus-induced asthma exacerbations. However, the underlying mechanisms of whether and how AdV affects asthmatic patients remains unclear. To address this question, we investigated nasal epithelial cells (NAEPCs) derived from a pediatric exacerbation study cohort for experimental analyses. We analyzed twenty-one different green-fluorescent protein- and luciferase-tagged AdV types in submerged 2D and organotypic 3D cell culture models. Transduction experiments revealed robust transduction of AdV type 5 (AdV5) in NAEPCs, which was associated with an increased uptake of AdV5 in the presence of HDM. In healthy and asthmatic NAEPCs exposed to HDM before infection, we observed a time- and dose-dependent increase of AdV5 uptake associated with upregulation of entry receptors for AdV5. Furthermore, electron microscopic and histologic analyses of 3D cell cultures revealed an impairment of the respiratory cilia after HDM exposition. This ex vivo pilot study shows the impact of AdV infection and HDM exposition in a primary cell culture model for asthma.
Keywords: CAR; CD46; adenovirus; allergy; asthma; house dust mite; pathogenesis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest. The authors have declared that they have no competing interests. This work has not been published before, and it is not under consideration for publication elsewhere. The manuscript has been approved for publication by all co-authors.
Figures





References
-
- Li L., Woo Y.Y., de Bruyne J.A., Nathan A.M., Kee S.Y., Chan Y.F., Chiam C.W., Eg K.P., Thavagnanam S., Sam I.C. Epidemiology, clinical presentation and respiratory sequelae of adenovirus pneumonia in children in Kuala Lumpur, Malaysia. PLoS ONE. 2018;13:e0205795. doi: 10.1371/journal.pone.0209720. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

