Small-Molecule Chemical Knockdown of MuRF1 in Melanoma Bearing Mice Attenuates Tumor Cachexia Associated Myopathy
- PMID: 33050629
- PMCID: PMC7600862
- DOI: 10.3390/cells9102272
Small-Molecule Chemical Knockdown of MuRF1 in Melanoma Bearing Mice Attenuates Tumor Cachexia Associated Myopathy
Abstract
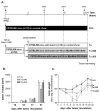
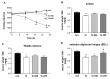
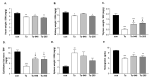
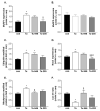
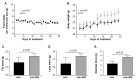
: Patients with malignant tumors frequently suffer during disease progression from a syndrome referred to as cancer cachexia (CaCax): CaCax includes skeletal muscle atrophy and weakness, loss of bodyweight, and fat tissues. Currently, there are no FDA (Food and Drug Administration) approved treatments available for CaCax. Here, we studied skeletal muscle atrophy and dysfunction in a murine CaCax model by injecting B16F10 melanoma cells into mouse thighs and followed mice during melanoma outgrowth. Skeletal muscles developed progressive weakness as detected by wire hang tests (WHTs) during days 13-23. Individual muscles analyzed at day 24 had atrophy, mitochondrial dysfunction, augmented metabolic reactive oxygen species (ROS) stress, and a catabolically activated ubiquitin proteasome system (UPS), including upregulated MuRF1. Accordingly, we tested as an experimental intervention of recently identified small molecules, Myomed-205 and -946, that inhibit MuRF1 activity and MuRF1/MuRF2 expression. Results indicate that MuRF1 inhibitor fed attenuated induction of MuRF1 in tumor stressed muscles. In addition, the compounds augmented muscle performance in WHTs and attenuated muscle weight loss. Myomed-205 and -946 also rescued citrate synthase and complex-1 activities in tumor-stressed muscles, possibly suggesting that mitochondrial-metabolic and muscle wasting effects in this CaCax model are mechanistically connected. Inhibition of MuRF1 during tumor cachexia may represent a suitable strategy to attenuate skeletal muscle atrophy and dysfunction.
Keywords: MuRF1; cancer cachexia; chemical biology; melanoma tumors; mitochondrial metabolism; muscle wasting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Baracos V.E., Martin L., Korc M., Guttridge D.C., Fearon K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Primers. 2018;4:17105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

