CASCADE: a phase 2, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the effect of tezepelumab on airway inflammation in patients with uncontrolled asthma
- PMID: 33050900
- PMCID: PMC7550845
- DOI: 10.1186/s12931-020-01513-x
CASCADE: a phase 2, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the effect of tezepelumab on airway inflammation in patients with uncontrolled asthma
Abstract
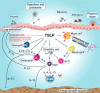
Background: Patients with severe, uncontrolled asthma, particularly those with a non-eosinophilic phenotype, have a great unmet need for new treatments that act on a broad range of inflammatory pathways in the airway. Tezepelumab is a human monoclonal antibody that blocks the activity of thymic stromal lymphopoietin, an epithelial cytokine. In the PATHWAY phase 2b study (NCT02054130), tezepelumab reduced exacerbations by up to 71% in adults with severe, uncontrolled asthma, irrespective of baseline eosinophilic inflammatory status. This article reports the design and objectives of the phase 2 CASCADE study.
Methods: CASCADE is an ongoing exploratory, phase 2, randomized, double-blind, placebo-controlled, parallel-group study aiming to assess the anti-inflammatory effects of tezepelumab 210 mg administered subcutaneously every 4 weeks for 28 weeks in adults aged 18-75 years with uncontrolled, moderate-to-severe asthma. The primary endpoint is the change from baseline to week 28 in airway submucosal inflammatory cells (eosinophils, neutrophils, T cells and mast cells) from bronchoscopic biopsies. Epithelial molecular phenotyping, comprising the three-gene-mean technique, will be used to assess participants' type 2 (T2) status to enable evaluation of the anti-inflammatory effect of tezepelumab across the continuum of T2 activation. Other exploratory analyses include assessments of the impact of tezepelumab on airway remodelling, including reticular basement membrane thickening and airway epithelial integrity. At the onset of the COVID-19 pandemic, the protocol was amended to address the possibility that site visits would be limited. The amendment allowed for: at-home dosing of study drug by a healthcare professional, extension of the treatment period by up to 6 months so patients are able to attend an onsite visit to undergo the end-of-treatment bronchoscopy, and replacement of final follow-up visits with a virtual or telephone visit.
Discussion: CASCADE aims to determine the mechanisms by which tezepelumab improves clinical asthma outcomes by evaluating the effect of tezepelumab on airway inflammatory cells and remodelling in patients with moderate-to-severe, uncontrolled asthma. An important aspect of this study is the evaluation of the anti-inflammatory effect of tezepelumab across patients with differing levels of eosinophilic and T2 inflammation.
Trial registration: NCT03688074 (ClinicalTrials.gov). Registered 28 September 2018.
Keywords: Asthma; T2 inflammation; TSLP; Tezepelumab.
Conflict of interest statement
CEB has received grants and consultancy fees from AstraZeneca. CE, GC, AM, CS and KB are employees of AstraZeneca and own stock and stock options in AstraZeneca. JD and JRP are employees of Amgen Inc. and own stock and stock options in Amgen Inc. SD and LC declare that they have no competing interests.
Figures

Similar articles
-
Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial.Lancet Respir Med. 2021 Nov;9(11):1299-1312. doi: 10.1016/S2213-2600(21)00226-5. Epub 2021 Jul 10. Lancet Respir Med. 2021. PMID: 34256031 Clinical Trial.
-
NAVIGATOR: a phase 3 multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the efficacy and safety of tezepelumab in adults and adolescents with severe, uncontrolled asthma.Respir Res. 2020 Oct 13;21(1):266. doi: 10.1186/s12931-020-01526-6. Respir Res. 2020. PMID: 33050934 Free PMC article. Clinical Trial.
-
Unmet need in severe, uncontrolled asthma: can anti-TSLP therapy with tezepelumab provide a valuable new treatment option?Respir Res. 2020 Oct 15;21(1):268. doi: 10.1186/s12931-020-01505-x. Respir Res. 2020. PMID: 33059715 Free PMC article. Review.
-
DESTINATION: a phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the long-term safety and tolerability of tezepelumab in adults and adolescents with severe, uncontrolled asthma.Respir Res. 2020 Oct 21;21(1):279. doi: 10.1186/s12931-020-01541-7. Respir Res. 2020. PMID: 33087119 Free PMC article. Clinical Trial.
-
Tezepelumab in patients with allergic and eosinophilic asthma.Allergy. 2024 May;79(5):1134-1145. doi: 10.1111/all.15986. Epub 2023 Dec 26. Allergy. 2024. PMID: 38146651 Review.
Cited by
-
Targeting TSLP in Asthma.J Asthma Allergy. 2022 Jun 3;15:749-765. doi: 10.2147/JAA.S275039. eCollection 2022. J Asthma Allergy. 2022. PMID: 35685846 Free PMC article. Review.
-
Novel potential treatable traits in asthma: Where is the research taking us?J Allergy Clin Immunol Glob. 2022 Apr 9;1(2):27-36. doi: 10.1016/j.jacig.2022.04.001. eCollection 2022 May. J Allergy Clin Immunol Glob. 2022. PMID: 37780590 Free PMC article. Review.
-
Efficacy and Potential Positioning of Tezepelumab in the Treatment of Severe Asthma.Open Respir Arch. 2023 Jan 3;5(2):100231. doi: 10.1016/j.opresp.2022.100231. eCollection 2023 Apr-Jun. Open Respir Arch. 2023. PMID: 37496871 Free PMC article. Review.
-
Targeting interleukin-33 and thymic stromal lymphopoietin pathways for novel pulmonary therapeutics in asthma and COPD.Eur Respir Rev. 2023 Jan 25;32(167):220144. doi: 10.1183/16000617.0144-2022. Print 2023 Mar 31. Eur Respir Rev. 2023. PMID: 36697211 Free PMC article. Review.
-
Guideline for the use of immunobiologicals in chronic rhinosinusitis with nasal polyps (CRSwNP) in Brazil.Braz J Otorhinolaryngol. 2022 May-Jun;88(3):471-480. doi: 10.1016/j.bjorl.2021.03.003. Epub 2021 Apr 3. Braz J Otorhinolaryngol. 2022. PMID: 33867274 Free PMC article. Review.
References
-
- Global strategy for asthma management and prevention. Updated 2019. [http://ginasthma.org/gina-reports/]. Accessed 21 Sept 2020.

