3D visualization of macromolecule synthesis
- PMID: 33051003
- PMCID: PMC7669265
- DOI: 10.7554/eLife.60354
3D visualization of macromolecule synthesis
Abstract
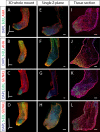
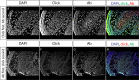
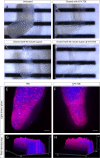
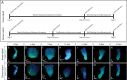
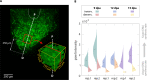
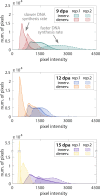
Measuring nascent macromolecular synthesis in vivo is key to understanding how cells and tissues progress through development and respond to external cues. Here we perform in vivo injection of alkyne- or azide-modified analogs of thymidine, uridine, methionine, and glucosamine to label nascent synthesis of DNA, RNA, protein, and glycosylation. Three-dimensional volumetric imaging of nascent macromolecule synthesis was performed in axolotl salamander tissue using whole-mount click chemistry-based fluorescent staining followed by light sheet fluorescent microscopy. We also developed an image processing pipeline for segmentation and classification of morphological regions of interest and individual cells, and we apply this pipeline to the regenerating humerus. We demonstrate our approach is sensitive to biological perturbations by measuring changes in DNA synthesis after limb denervation. This method provides a powerful means to quantitatively interrogate macromolecule synthesis in heterogenous tissues at the organ, cellular, and molecular levels of organization.
Keywords: Axolotl; click-chemistry; developmental biology; light sheet fluorescence microscopy; macromolecules; regenerative medicine; stem cells; whole mount.
Plain language summary
Cells often respond to changes in their environment by producing new molecules and building new cell components, such as proteins, which perform most tasks in the cell, or DNA and RNA, which carry genetic information. Complex tissues – such as limbs, which are made up of muscles, tendons, bones and cartilage – are difficult to see through, so studying when and where cells in these tissues produce different types of molecules is challenging. New approaches combining advanced three-dimensional microscopy and fluorescent labelling of molecules could provide a way to study these processes within whole animal tissues. One application for this is studying how salamanders regrow lost limbs. When salamanders such as axolotls regrow a limb, some cells in the limb stump form a group called the blastema. The blastema contains cells that are specialized to different purposes. Each cell in the blastema produces many new proteins as well as new DNA and RNA molecules. Fluorescently labeling particular molecules and taking images of the regenerating limb at different times can help to reveal how these new molecules control and coordinate limb regrowth. Duerr et al. developed a three-dimensional microscopy technique to study the production of new molecules in regenerating axolotl limbs. The method labeled molecules of different types with fluorescent markers. As a result, new proteins, RNA and DNA glowed under different colored lights. Duerr et al. used their method to show that nerve damage, which hinders limb regrowth in salamanders, reduces DNA production in the blastema. There are many possible applications of this microscopy method. Since the technique allows the spatial arrangement of the cells and molecules studied to be preserved, it makes it possible to investigate which molecules each cell is making and how they interact across a tissue. Not only does the technique have the potential to reveal much more about limb regrowth at all stages, but the fluorescent markers used can also be easily adapted to many other applications.
© 2020, Duerr et al.
Conflict of interest statement
TD, EC, EJ, JF, MJ, JG, SS, JM No competing interests declared
Figures






References
-
- Chung K, Wallace J, Kim SY, Kalyanasundaram S, Andalman AS, Davidson TJ, Mirzabekov JJ, Zalocusky KA, Mattis J, Denisin AK, Pak S, Bernstein H, Ramakrishnan C, Grosenick L, Gradinaru V, Deisseroth K. Structural and molecular interrogation of intact biological systems. Nature. 2013;497:332–337. doi: 10.1038/nature12107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

