Thirteen-year analyses of medical oncology outpatient day clinic data: a changing field
- PMID: 33051192
- PMCID: PMC7555099
- DOI: 10.1136/esmoopen-2020-000880
Thirteen-year analyses of medical oncology outpatient day clinic data: a changing field
Abstract
Background: Novel treatment modalities like targeted therapy and immunotherapy are currently changing treatment strategies and protocols in the field of medical oncology.
Methods: Numbers of patients and patient contacts admitted to medical oncology day clinics of a large European academic cancer centre in the period from 2006 to 2018 were analysed using our patient administration system.
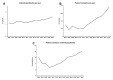
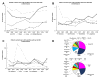
Results: A patient cohort of 9.870 consecutive individual patients with 125.679 patient contacts was descriptively and retrospectively characterised. Mean age was 59.9 years. A substantial increase in both individual patients treated per year (+45.4%; 2006: 1.100; 2018: 1.599) and annual patient contacts (+63.3%; 2006: 8.857; 2018: 14.467) between 2006 and 2018 was detected. Hence and most interestingly, the ratio of visits per patient increased by approximately one visit per patient per year over the last 12 years (+12.4%; 2006: 8.0; 2018: 9.0). Further, a decrease of patient contacts in more prevalent entities like breast cancer was found, while contacts for orphan diseases like myeloma and sarcoma increased substantially. Interestingly, female patients showed more per patient contacts as compared with men (13.5 vs 11.9). Lastly, short-term safety data of outpatient day clinic admissions are reported.
Conclusions: We present a representative and large set of patient contacts over time that indicates an increasing load in routine clinical work of outpatient cancer care. Increases observed were highest for orphan diseases, likely attributed to centralisation effects and increased treatment complexity.
Keywords: cancer treatment; chemotherapy; oncology; outpatient treatment; targeted therapy.
© Author (s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. Published by BMJ on behalf of the European Society for Medical Oncology.
Conflict of interest statement
Competing interests: MM has received honoraria for lectures and consultation from Roche, Novartis, Eli-Lilly and Medmedia and travel support from Amgen, Roche, Novartis, Pierre Fabre, Daiichi Sankyo and Eisai. RB was an advisor for Astra-Zeneca, Celgene, Daiichi, Eisai, Eli-Lilly, MSD, Novartis, Pfizer, Pierre-Fabre, Puma, Roche and Samsung and received lecture honoraria from Accord, Astra-Zeneca, BMS, Celgene, Eli-Lilly, Novartis, Pfizer, Pierre-Fabre, Roche, Sandoz as well as research support from Daiichi, Novartis and Roche. MP has received honoraria for lectures, consultation or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, Tocagen. The following for-profit companies have supported clinical trials and contracted research conducted by MP with payments made to his institution: Böhringer-Ingelheim, Bristol-Myers Squibb, Roche, Daiichi Sankyo, Merck Sharp & Dome, Novocure, GlaxoSmithKline, AbbVie. ASB has research support from Daiichi Sankyo (≤ €10 000), Roche (> €10 000) and honoraria for lectures, consultation or advisory board participation from Roche Bristol-Meyers Squibb, Merck, Daiichi Sankyo (all <€5000) as well as travel support from Roche, Amgen and AbbVie. TB reports personal fees from Bayer (lecture fee, advisory board), personal fees from PharmaMar (lecture fee, advisory board), personal fees from Eli-Lilly (lecture fee, advisory board) outside the submitted work. SZ-M received honoraria for advisory boards and/or lectures from Boehringer Ingelheim, Merck Sharp & Dohme, Bristol Myers Squibb, Roche, AstraZeneca, Takeda and Pfizer. Research support to her was granted by Merck Sharp & Dohme. TF hast received honoraria from MSD, Merck Darmstadt, Roche, BMS, Accord, Sanofi, Boehringer Ingelheim, Novartis and was and advisor or consultant for MSD; Merck Darmstadt, Amgen, Pfizer, Sanofi and Boehringer Ingelheim. He has further received travel expenses, including accommodations, from Roche, MS and BMS as well as research grants or funding from MSD, Merck Darmstadt. AI-M announces following COIs: Participation at advisory boards of MSD, Servier and BMS, lecture honoraria from Eli Lilly and Servier and travel support from Roche and BMS. She has also served as local PI for clinical trials sponsored by BMS and Astellas. All other authors report no relevant conflicts of interest.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

