Sex-Specific Vasopressin Signaling Buffers Stress-Dependent Synaptic Changes in Female Mice
- PMID: 33051356
- PMCID: PMC7659452
- DOI: 10.1523/JNEUROSCI.1026-20.2020
Sex-Specific Vasopressin Signaling Buffers Stress-Dependent Synaptic Changes in Female Mice
Abstract
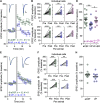
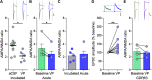
In many species, social networks provide benefit for both the individual and the collective. In addition to transmitting information to others, social networks provide an emotional buffer for distressed individuals. Our understanding about the cellular mechanisms that contribute to buffering is poor. Stress has consequences for the entire organism, including a robust change in synaptic plasticity at glutamate synapses onto corticotropin-releasing hormone (CRH) neurons in the paraventricular nucleus of the hypothalamus (PVN). In females, however, this stress-induced metaplasticity is buffered by the presence of a naive partner. This buffering may be because of discrete behavioral interactions, signals in the context in which the interaction occurs (i.e., olfactory cues), or it may be influenced by local signaling events in the PVN. Here, we show that local vasopressin (VP) signaling in PVN buffers the short-term potentiation (STP) at glutamate synapses after stress. This social buffering of metaplasticity, which requires the presence of another individual, was prevented by pharmacological inhibition of the VP 1a receptor (V1aR) in female mice. Exogenous VP mimicked the effects of social buffering and reduced STP in CRHPVN neurons from females but not males. These findings implicate VP as a potential mediator of social buffering in female mice.SIGNIFICANCE STATEMENT In many organisms, including rodents and humans, social groups are beneficial to overall health and well-being. Moreover, it is through these social interactions that the harmful effects of stress can be mitigated, a phenomenon known as social buffering. In the present study, we describe a critical role for the neuropeptide vasopressin (VP) in social buffering of synaptic metaplasticity in stress-responsive corticotropin-releasing hormone (CRH) neurons in female mice. These effects of VP do not extend to social buffering of stress behaviors, suggesting this is a very precise and local form of sex-specific neuropeptide signaling.
Keywords: CRH; hypothalamus; social; stress; synapse; vasopressin.
Copyright © 2020 the authors.
Figures




References
-
- Bangasser DA, Curtis A, Reyes BAS, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ (2010) Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 15:877–904. 10.1038/mp.2010.89 - DOI - PMC - PubMed
-
- Biag J, Huang Y, Gou L, Hintiryan H, Askarinam A, Hahn JD, Toga AW, Dong H-W (2012) Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: a study of immunostaining and multiple fluorescent tract tracing. J Comp Neurol 520:6–33. 10.1002/cne.22698 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
