Sarcomatoid renal cell carcinoma: biology, natural history and management
- PMID: 33051619
- PMCID: PMC7551522
- DOI: 10.1038/s41585-020-00382-9
Sarcomatoid renal cell carcinoma: biology, natural history and management
Abstract
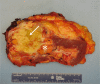
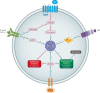
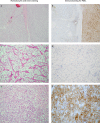
Sarcomatoid dedifferentiation is an uncommon feature that can occur in most histological subtypes of renal cell carcinomas (RCCs) and carries a decidedly poor prognosis. Historically, conventional treatments for sarcomatoid RCCs (sRCCs) have shown little efficacy, and median survival is commonly 6-13 months. Despite being first described in 1968, the mechanisms driving sarcomatoid dedifferentiation remain poorly understood, and information and treatment options available to physicians and patients are limited. When diagnosed at an early stage, surgical intervention remains the treatment of choice. However, preoperative identification through routine imaging or biopsy is unreliable and most patients present with advanced disease and systemic symptoms. For these patients, the role of cytoreductive nephrectomy is disputed. The expansion of immunotherapies approved for RCCs has generated a search for biomarkers that might be indicative of treatment response in sRCCs, although a proven effective systemic agent remains elusive. PDL1 expression is increased in sarcomatoid dedifferentiated renal tumours, which suggests that patients with sRCCs could benefit from PD1 and/or PDL1 immune checkpoint blockade therapy. Treatment outcomes for sarcomatoid tumours have remained relatively consistent compared with other RCCs, but further investigation of the tumour-immune cell microenvironment might yield insights into further therapeutic possibilities.
Conflict of interest statement
J.A.K. is a member of consulting or advisory boards for, and has received honoraria from, Merck, EMD Serono, Genentech, Novartis and Pfizer. J.A.K. has received institutional research funding from Merck, Genentech and Mirati. R.J.M. has received grants and paid consultancy from Eisai, Exelixis, Genentech, Roche, Novartis, Merck and Pfizer, paid consultancy from Astra Zeneca and grants from Bristol Myers Squibb. All other authors declare no competing interests.
Figures

References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69:7–34. - PubMed
-
- Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. - PubMed
-
- Motzer RJ, et al. Kidney cancer, version 2.2017, NCCN clinical practice guidelines in oncology. J. Natl Compr. Canc. Netw. 2017;15:804–834. - PubMed
-
- Delahunt B. Sarcomatoid renal carcinoma: the final common dedifferentiation pathway of renal epithelial malignancies. Pathology. 1999;31:185–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

