Therapeutic Drug Monitoring of Tumor Necrosis Factor Antagonists in Crohn Disease: A Theoretical Construct to Apply Pharmacokinetics and Guidelines to Clinical Practice
- PMID: 33051647
- PMCID: PMC8314098
- DOI: 10.1093/ibd/izaa265
Therapeutic Drug Monitoring of Tumor Necrosis Factor Antagonists in Crohn Disease: A Theoretical Construct to Apply Pharmacokinetics and Guidelines to Clinical Practice
Abstract
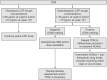
Therapeutic drug monitoring (TDM) is the measurement of drug and antidrug antibody concentrations in individuals to guide treatment decisions. In patients with Crohn disease (CD), TDM, used either reactively or proactively, is emerging as a valuable tool for optimization of tumor necrosis factor (TNF) antagonist therapy. Reactive TDM is carried out in response to treatment failure, whereas proactive TDM involves the periodic monitoring of patients responding to TNF antagonist therapy to allow treatment optimization. In patients with CD, most of the available data for TDM relate to the first-to-market TNF antagonist infliximab and, to a lesser extent, to adalimumab and certolizumab pegol. Several gastroenterology associations, including the American Gastroenterology Association, have endorsed the use of reactive TDM in patients with active CD. However, fewer recommendations currently exist for the use of proactive TDM, although several new prospective randomized controlled trials evaluating proactive TDM strategies have been published. In this review, the current evidence for reactive and proactive TDM is discussed, and a proactive treatment algorithm for certolizumab pegol based on previously published threshold concentrations is proposed.
Keywords: antidrug antibody; certolizumab pegol; proactive TDM; reactive TDM; therapeutic drug monitoring.
© 2020 Crohn’s & Colitis Foundation. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation.
Figures

References
-
- Lin K, Mahadevan U. Pharmacokinetics of biologics and the role of therapeutic monitoring. Gastroenterol Clin North Am. 2014;43:565–579. - PubMed
-
- Vande Casteele N, Gils A. Pharmacokinetics of anti-TNF monoclonal antibodies in inflammatory bowel disease: adding value to current practice. J Clin Pharmacol. 2015;55:S39–S50. - PubMed
-
- Vande Casteele N, Ferrante M, Van Assche G, et al. . Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148:1320–132 9.e3. - PubMed
-
- Lab Corp. Certolizumab and anti-certolizumab antibody, DoseASSURE™ CTZ. Accessed December 31, 2019. https://www.labcorp.com/test-menu/46121/certolizumab-and-anti-certolizum....
-
- Mayo Clinic Laboratories. Test ID: FCERT–certolizumab pegol and anti-certolizumab antibodies, serum. Accessed December 31, 2019. https://qa.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/75198.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

