Comparative docking studies to understand the binding affinity of nicotine with soluble ACE2 (sACE2)-SARS-CoV-2 complex over sACE2
- PMID: 33052306
- PMCID: PMC7543737
- DOI: 10.1016/j.toxrep.2020.10.002
Comparative docking studies to understand the binding affinity of nicotine with soluble ACE2 (sACE2)-SARS-CoV-2 complex over sACE2
Abstract
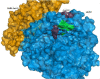
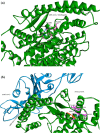
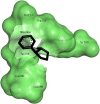
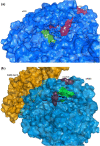
The study aimed to validate the proficiency of nicotine binding with the soluble angiotensin-converting enzyme II receptor (sACE2) with or without SARS-CoV-2 in the context of its binding affinity. Modelled human sACE2 and the spike (S1) protein of Indian SARS-CoV-2 (INS1) docked with each other. On the other hand, nicotine docked with sACE2 in the presence or absence of SARS-CoV-2. Nicotine established a stable interaction with negatively charged Asp368 of sACE2, which in turn binds with amino acids like Thr362, Lys363, Thr365, Thr371, and Ala372. In the presence of nicotine, INS1 and sACE2 showed a reduced binding affinity score of -12.6 kcal/mol (Vs -15.7 kcal/mol without nicotine), and a lowered interface area of 1933.6 Å2 (Vs 2057.3Å2 without nicotine). The neuronal nicotinic acetylcholine receptor (nN-AChR) and angiotensin-converting enzyme 2 (ACE2) receptor showed 19.85% sequence identity among themselves. Following these receptors possessed conserved Trp302 and Cys344 amino acids between them for nicotine binding. However, nicotine showed a higher binding affinity score of -6.33 kcal/mol for the sACE2-INS1 complex than the sACE2 alone with -5.24 kcal/mol. A lowered inhibitory constant value of 22.95μM recorded while nicotine interacted with the sACE2-INS1 complex over the sACE2 alone with 151.69 μM. In summary, nicotine showed a profound binding affinity for the sACE2-INS1 complex than the sACE2 alone paving for the clinical trials to validate its therapeutic efficacy as a bitter compound against the SARS-CoV-2 virulence.
Keywords: Neuronal nicotinic acetylcholine receptor; Nicotine; SARS-CoV-2; Smokers; Soluble ACE2; sACE2-INS1 complex.
© 2020 The Authors.
Conflict of interest statement
The authors report no declarations of interest.
Figures



Similar articles
-
Determination of soluble angiotensin-converting enzyme 2 in saliva samples and its association with nicotine.Environ Res. 2023 Jan 1;216(Pt 1):114443. doi: 10.1016/j.envres.2022.114443. Epub 2022 Oct 3. Environ Res. 2023. PMID: 36195157 Free PMC article.
-
Determinants of soluble angiotensin-converting enzyme 2 concentrations in adult patients with complex congenital heart disease.Clin Res Cardiol. 2022 Feb;111(2):154-162. doi: 10.1007/s00392-020-01782-y. Epub 2020 Dec 5. Clin Res Cardiol. 2022. PMID: 33280062 Free PMC article.
-
Protein Scaffold-Based Multimerization of Soluble ACE2 Efficiently Blocks SARS-CoV-2 Infection In Vitro and In Vivo.Adv Sci (Weinh). 2022 Sep;9(27):e2201294. doi: 10.1002/advs.202201294. Epub 2022 Jul 27. Adv Sci (Weinh). 2022. PMID: 35896894 Free PMC article.
-
Novel sACE2-Anti-CD16VHH Fusion Protein Surreptitiously Inhibits SARS-CoV-2 Variant Spike Proteins and Macrophage Cytokines, and Activates Natural Killer Cell Cytotoxicity.Vaccines (Basel). 2025 Feb 17;13(2):199. doi: 10.3390/vaccines13020199. Vaccines (Basel). 2025. PMID: 40006745 Free PMC article.
-
Soluble Angiotensin-Converting Enzyme 2 as a Prognostic Biomarker for Disease Progression in Patients Infected with SARS-CoV-2.Diagnostics (Basel). 2022 Apr 1;12(4):886. doi: 10.3390/diagnostics12040886. Diagnostics (Basel). 2022. PMID: 35453934 Free PMC article.
Cited by
-
Targeting ACE2 for COVID-19 Therapy: Opportunities and Challenges.Am J Respir Cell Mol Biol. 2021 Apr;64(4):416-425. doi: 10.1165/rcmb.2020-0322PS. Am J Respir Cell Mol Biol. 2021. PMID: 33296619 Free PMC article. Review.
-
COVID-19-A Theory of Autoimmunity Against ACE-2 Explained.Front Immunol. 2021 Mar 23;12:582166. doi: 10.3389/fimmu.2021.582166. eCollection 2021. Front Immunol. 2021. PMID: 33833750 Free PMC article. Review.
-
Fraxinol attenuates LPS-induced acute lung injury by equilibrating ACE-Ang II-AT1R and ACE2-Ang (1-7)-Mas and inhibiting NLRP3.Pharm Biol. 2022 Dec;60(1):979-989. doi: 10.1080/13880209.2022.2067571. Pharm Biol. 2022. PMID: 35588103 Free PMC article.
-
Nicotinic cholinergic system and COVID-19: In silico identification of interactions between α7 nicotinic acetylcholine receptor and the cryptic epitopes of SARS-Co-V and SARS-CoV-2 Spike glycoproteins.Food Chem Toxicol. 2021 Mar;149:112009. doi: 10.1016/j.fct.2021.112009. Epub 2021 Jan 24. Food Chem Toxicol. 2021. PMID: 33503469 Free PMC article.
-
The naturally-derived alkaloids as a potential treatment for COVID-19: A scoping review.Phytother Res. 2022 Jul;36(7):2686-2709. doi: 10.1002/ptr.7442. Epub 2022 Mar 30. Phytother Res. 2022. PMID: 35355337 Free PMC article.
References
-
- Meo S.A., Alhowikan A.M., Al-Khlaiwi T., Meo I.M., Halepoto D.M., Iqbal M. Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur. Rev. Med. Pharmacol. Sci. 2020;24(4):2012–2019. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

