This is a preprint.
Alpha 1 Antitrypsin is an Inhibitor of the SARS-CoV-2-Priming Protease TMPRSS2
- PMID: 33052338
- PMCID: PMC7553163
- DOI: 10.1101/2020.05.04.077826
Alpha 1 Antitrypsin is an Inhibitor of the SARS-CoV-2-Priming Protease TMPRSS2
Update in
-
Alpha 1 Antitrypsin is an Inhibitor of the SARS-CoV-2-Priming Protease TMPRSS2.Pathog Immun. 2021 Apr 26;6(1):55-74. doi: 10.20411/pai.v6i1.408. eCollection 2021. Pathog Immun. 2021. PMID: 33969249 Free PMC article.
Abstract
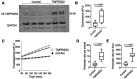
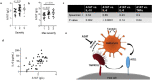
Host proteases have been suggested to be crucial for dissemination of MERS, SARS-CoV, and SARS-CoV-2 coronaviruses, but the relative contribution of membrane versus intracellular proteases remains controversial. Transmembrane serine protease 2 (TMPRSS2) is regarded as one of the main proteases implicated in the coronavirus S protein priming, an important step for binding of the S protein to the angiotensin-converting enzyme 2 (ACE2) receptor before cell entry. The main cellular location where the SARS-CoV-2 S protein priming occurs remains debatable, therefore hampering the development of targeted treatments. Herein, we identified the human extracellular serine protease inhibitor (serpin) alpha 1 antitrypsin (A1AT) as a novel TMPRSS2 inhibitor. Structural modeling revealed that A1AT docked to an extracellular domain of TMPRSS2 in a conformation that is suitable for catalysis, resembling similar serine protease-inhibitor complexes. Inhibitory activity of A1AT was established in a SARS-CoV-2 viral load system. Notably, plasma A1AT levels were associated with COVID-19 disease severity. Our data support the key role of extracellular serine proteases in SARS-CoV-2 infections and indicate that treatment with serpins, particularly the FDA-approved drug A1AT, may be effective in limiting SARS-CoV-2 dissemination by affecting the surface of the host cells.
Summary: Delivery of extracellular serine protease inhibitors (serpins) such as A1AT has the capacity to reduce SARS-CoV-2 dissemination by binding and inhibiting extracellular proteases on the host cells, thus, inhibiting the first step in SARS-CoV-2 cell cycle (i.e. cell entry).
Conflict of interest statement
Conflict of interest
M.E.R. is a consultant for Pulm One, Spoon Guru, ClostraBio, Serpin Pharma, Celgene, Astra Zeneca, Allakos, Arena Pharmaceuticals, Guidepoint, and Suvretta Capital Management and has an equity interest in the first four listed and royalties from reslizumab (Teva Pharmaceuticals), PEESSv2 (Mapi Research Trust), and UpToDate. M.E.R. is an inventor of patents owned by Cincinnati Children’s Hospital. M.E.R. and N.P.A. are inventors of a patent owned by Cincinnati Children’s Hospital with the provisional number of 63/017,027.
Figures



References
-
- Bhattacharyya C., Das C., Ghosh A., Singh A. K., Mukherjee S., Majumder P. P., Basu A., Biswas N. K.. 2020. Global Spread of SARS-CoV-2 Subtype with Spike Protein Mutation D614G is Shaped by Human Genomic Variations that Regulate Expression of TMPRSS2 and MX1 Genes. BioRxiv
-
- Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y., Men D., Huang Q., Liu Y., Yang B., Ding J., and Li F.. 2020. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
