This is a preprint.
TMEM41B is a pan-flavivirus host factor
- PMID: 33052348
- PMCID: PMC7553181
- DOI: 10.1101/2020.10.09.334128
TMEM41B is a pan-flavivirus host factor
Update in
-
TMEM41B Is a Pan-flavivirus Host Factor.Cell. 2021 Jan 7;184(1):133-148.e20. doi: 10.1016/j.cell.2020.12.005. Epub 2020 Dec 9. Cell. 2021. PMID: 33338421 Free PMC article.
Abstract
Flaviviruses pose a constant threat to human health. These RNA viruses are transmitted by the bite of infected mosquitoes and ticks and regularly cause outbreaks. To identify host factors required for flavivirus infection we performed full-genome loss of function CRISPR-Cas9 screens. Based on these results we focused our efforts on characterizing the roles that TMEM41B and VMP1 play in the virus replication cycle. Our mechanistic studies on TMEM41B revealed that all members of the Flaviviridae family that we tested require TMEM41B. We tested 12 additional virus families and found that SARS-CoV-2 of the Coronaviridae also required TMEM41B for infection. Remarkably, single nucleotide polymorphisms (SNPs) present at nearly twenty percent in East Asian populations reduce flavivirus infection. Based on our mechanistic studies we hypothesize that TMEM41B is recruited to flavivirus RNA replication complexes to facilitate membrane curvature, which creates a protected environment for viral genome replication.
Highlights: TMEM41B and VMP1 are required for both autophagy and flavivirus infection, however, autophagy is not required for flavivirus infection.TMEM41B associates with viral proteins and likely facilitates membrane remodeling to establish viral RNA replication complexes.TMEM41B single nucleotide polymorphisms (SNPs) present at nearly twenty percent in East Asian populations reduce flavivirus infection.TMEM41B-deficient cells display an exaggerated innate immune response upon high multiplicity flavivirus infection.
Conflict of interest statement
DECLARATION OF INTERESTS
C. M. Rice is a founder of Apath LLC, a Scientific Advisory Board member of Imvaq Therapeutics, Vir Biotechnology, and Arbutus Biopharma, and an advisor for Regulus Therapeutics and Pfizer. The remaining authors declare no competing interests. C. M. Rudin serves on the Scientific Advisory Boards of Bridge Medicines, Earli, and Harpoon Therapeutics.
Figures




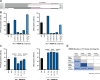
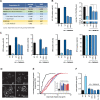
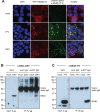
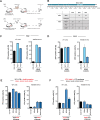

References
-
- Yellow fever in Africa and the Americas, 2016. Releve epidemiologique hebdomadaire 92, 442–452. - PubMed
-
- Ahmed Q.A., and Memish Z.A. (2017). Yellow fever from Angola and Congo: a storm gathers. Tropical doctor 47, 92–96. - PubMed
-
- Baggen J., Persoons L., Jansen S., Vanstreels E., Jacquemyn M., Jochmans D., Neyts J., Dallmeier K., Maes P., and Daelemans D. (2020). Identification of TMEM106B as proviral host factor for SARS-CoV-2. bioRxiv. doi: 10.1101/2020.09.28.316281 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
