Effect of Risankizumab on Patient-Reported Outcomes in Moderate to Severe Psoriasis: The UltIMMa-1 and UltIMMa-2 Randomized Clinical Trials
- PMID: 33052382
- PMCID: PMC7557488
- DOI: 10.1001/jamadermatol.2020.3617
Effect of Risankizumab on Patient-Reported Outcomes in Moderate to Severe Psoriasis: The UltIMMa-1 and UltIMMa-2 Randomized Clinical Trials
Abstract
Importance: Demonstrating the value of therapies from a patient's perspective is increasingly important for patient-centered care.
Objective: To compare patient-reported outcomes (PROs) with risankizumab vs ustekinumab and placebo in psoriasis symptoms, health-related quality of life (HRQL), and mental health among patients with moderate to severe psoriasis.
Design, setting, and participants: The UltIMMa-1 and UltIMMa-2 studies were replicate 52-week phase 3, randomized, multisite, double-blind, placebo-controlled and active comparator-controlled trials conducted in 139 sites (including hospitals, academic medical centers, clinical research units, and private practices) globally in Asia-Pacific, Japan, Europe, and North America. Adults (≥18 years) with moderate to severe chronic plaque psoriasis with body surface area (BSA) involvement of 10% or more, Psoriasis Area Severity Index (PASI) scores of 12 or higher, and static Physician's Global Assessment (sPGA) scores of 3 or higher were included.
Interventions: In each trial, patients were randomly assigned (3:1:1) to 150 mg of risankizumab, 45 mg or 90 mg of ustekinumab (weight-based per label) for 52 weeks, or matching placebo for 16 weeks followed by risankizumab.
Main outcomes and measures: Integrated data from 2 trials were used to compare Psoriasis Symptom Scale (PSS) (total score and item scores for pain, redness, itchiness, and burning), Dermatology Life Quality Index (DLQI), 5-level EuroQoL-5D (EQ-5D-5L), and Hospital Anxiety and Depression Scale (HADS), at baseline, week 16, and week 52.
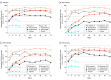
Results: A total of 997 patients with moderate to severe chronic plaque psoriasis were analyzed. Across all arms, the mean age was 47.2 to 47.8 years and 68.3% (136/199 for ustekinumab) to 73.0% (146/200 for placebo) were men. Patients' characteristics and PROs were comparable across all treatment arms at baseline (n = 598, 199, 200 for risankizumab, ustekinumab, and placebo, respectively). At week 16, a significantly greater proportion of patients treated with risankizumab than those treated with ustekinumab or placebo achieved PSS = 0, indicating no psoriasis symptoms (30.3% [181/598], 15.1% [30/199], 1.0% [2/200], both P < .001), and DLQI = 0 or 1 indicating no impact on skin-related HRQL (66.2%, 44.7%, 6.0%, P < .001). Significantly greater proportions of patients treated with risankizumab achieved minimally clinically important difference (MCID) than ustekinumab or placebo for DLQI (94.5% [516/546], 85.1% [149/175], 35.6% [64/180]; both P < .001), EQ-5D-5L (41.7% [249/597] vs 31.5% [62/197], P = .01; vs 19.0% [38/200], P < .001), and HADS (anxiety: 69.1% [381/551] vs 57.1% [104/182], P = .004; vs 35.9% [66/184], P < .001; depression: 71.1% [354/598] vs 60.4% [96/159], P = .01; vs 37.1% [59/159], P < .001). At week 52, improvements in patients treated with risankizumab compared with those treated with ustekinumab were sustained for PSS, DLQI, and EQ-5D-5L.
Conclusions and relevance: Risankizumab significantly improved symptoms of moderate to severe psoriasis, improved HRQL, and reduced psychological distress compared with ustekinumab or placebo.
Trial registration: ClinicalTrials.gov Identifiers: NCT02684370 (UltIMMa-1) and NCT02684357 (UltIMMa-2).
Conflict of interest statement
Figures

References
-
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70(5):871-881.e871-830. - PubMed
-
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team . Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377-385. doi: 10.1038/jid.2012.339 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

