Lanreotide autogel/depot in advanced enteropancreatic neuroendocrine tumours: final results of the CLARINET open-label extension study
- PMID: 33052555
- PMCID: PMC7881960
- DOI: 10.1007/s12020-020-02475-2
Lanreotide autogel/depot in advanced enteropancreatic neuroendocrine tumours: final results of the CLARINET open-label extension study
Abstract
Purpose: In the phase III CLARINET study (NCT00353496), lanreotide autogel/depot (lanreotide) significantly improved progression-free survival (PFS) vs placebo in patients with non-functioning intestinal or pancreatic neuroendocrine tumours (NETs). The aim of CLARINET open-label extension (OLE) (NCT00842348) was to evaluate long-term safety and efficacy of lanreotide in these patients.
Methods: Patients from the CLARINET study were eligible for the OLE if they had stable disease (irrespective of treatment group) or progressive disease (PD) (placebo-treated patients only). All patients in the OLE received lanreotide 120 mg every 28 days. Computed tomography or magnetic resonance imaging scans were conducted every 6 months and assessed locally for PD (the final scan was also assessed centrally).
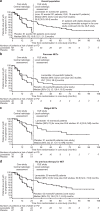
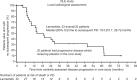
Results: Overall, 89 patients took part in the OLE (lanreotide, n = 42; placebo, n = 47). Median (range) exposure to lanreotide in patients who received lanreotide in the core study and OLE (LAN-LAN group) was 59.0 (26.0-102.3) months. In this group, the overall incidences of adverse events (AEs) and treatment-related AEs were lower in the OLE than in the core study. Median [95% CI] PFS in the LAN-LAN group was 38.5 [30.9; 59.4] months. In placebo-treated patients with PD at the end of the core study, time to death or subsequent PD during the OLE was 19 [10.1; 26.7] months.
Conclusions: This study provides new evidence on the long-term safety profile and sustained anti-tumour effects of lanreotide autogel/depot in indolent and progressive metastatic intestinal or pancreatic NETs.
Keywords: Lanreotide autogel; Lanreotide depot; Neuroendocrine tumours; Progression-free survival; Safety.
Conflict of interest statement
M.E.C.: Advisory board and speaker honoraria—Ipsen, Novartis, AAA, ITM and Pfizer. M.P.: Honoraria for presentation and advisory boards—Ipsen; research grants—Ipsen to former institution (Charité University Medicine, Berlin). A.T.P.: Research funding—Ipsen, Sanofi and Incyte; consulting/advisory fees—Ipsen and Roche; speaker fees—Lexicon, Novartis and Ipsen. JBC: Research funding and travel grant—Ipsen. E.S.: Travel grants and speaker fees—Ipsen and Novartis. E.M.W.: Consultancy/advisory fees—Ipsen, Novartis, Lexicon and Progenics. P.R.: Research funding—Ipsen; speaker fees – Ipsen, Novartis, AAA, ITM; consultancy—Ipsen, Novartis, AAA, ITM. X-MTT: Ipsen employee.
Figures

References
-
- Caplin ME, Pavel M, Cwikla JB, Phan AT, Raderer M, Sedlackova E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P, Investigators C. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–233. doi: 10.1056/NEJMoa1316158. - DOI - PubMed
-
- Ipsen Biopharmaceuticals, I.: Somatuline Depot Prescribing Information. https://www.ipsen.com/websites/Ipsen_Online/wp-content/uploads/sites/9/2... (2019). Accessed Jul 2019
-
- Ipsen Ltd.: Somatuline® Autogel® summary of product characteristics. https://www.medicines.org.uk/emc/product/8257/smpc (2019). Accessed Dec 2019
-
- Caplin ME, Pavel M, Cwikla JB, Phan AT, Raderer M, Sedlackova E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Gomez-Panzani E, Ruszniewski P, Investigators C. Anti-tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: the CLARINET open-label extension study. Endocr Relat Cancer. 2016;23(3):191–199. doi: 10.1530/ERC-15-0490. - DOI - PMC - PubMed
-
- WMA. Declaration of Helsinki Ethical principles for medical research involving human subjects. J Indian Med Assoc. 2009;107(6):403–405. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

