Preoperative molecular testing in thyroid nodules with Bethesda VI cytology: Clinical experience and review of the literature
- PMID: 33052631
- PMCID: PMC7983887
- DOI: 10.1002/dc.24637
Preoperative molecular testing in thyroid nodules with Bethesda VI cytology: Clinical experience and review of the literature
Abstract
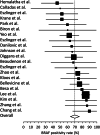
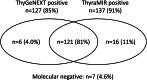
Risk assessment is critical to determine the timing of elective surgeries and preserve valuable resources in time of pandemic. This study was undertaken to better understand the potential value of molecular testing to risk-stratify thyroid nodules with malignant cytology (Bethesda VI). Systematic review of the literature contributed 21 studies representing 2036 preoperative specimens. The BRAF p.V600E substitution was detected in 46% to 90% of cases with a pooled positivity rate of 70% (95% confidence intervals: 64%-76%). None of the studies used comprehensive oncogene panels. Retrospective analysis of 531 clinical specimens evaluated with the next-generation sequencing ThyGeNEXT Thyroid Oncogene Panel identified a total of 436 gene alterations. BRAF mutation rate was 64% in specimens tested as part of standard clinical care and 75% in specimens from cross-sectional research studies (P = .022). Testing for additional actionable gene alterations such as TERT promoter mutations or RET and NTRK gene rearrangements further increased the diagnostic yield to 78%-85% and up to 95% when including the ThyraMIR Thyroid miRNA Classifier. These data support the role of molecular cytopathology in surgical and therapeutic decision-making and warrant additional studies.
Keywords: BRAF; miRNA; molecular testing; risk assessment; thyroid nodule.
© 2020 The Authors. Diagnostic Cytopathology published by Wiley Periodicals LLC.
Conflict of interest statement
E.L. is a consultant for Interpace Biosciences Inc.
Figures
References
-
- American Academy of Otolaryngology–Head and Neck Surgery . Guidance for Return to Practice for Otolaryngology‐Head and Neck Surgery. Accessed August 26, 2020. https://www.entnet.org/content/guidance-return-practice-otolaryngology-h...
-
- Society of Surgical Oncology . Resource for Management Options of Endocrine/Head and Neck Cancer During COVID‐19. Accessed August 26, 2020. https://www.surgonc.org/wp-content/uploads/2020/03/Endocrine-Head-and-Ne...
-
- American College of Surgeons . Roadmap for Resuming Elective Surgery after COVID‐19 Pandemic. Accessed August 26, 2020. https://www.facs.org/covid-19/clinical-guidance/roadmap-elective-surgery
-
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1‐133. 10.1089/thy.2015.0020. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

