First-in-Human Phase I Study of Iadademstat (ORY-1001): A First-in-Class Lysine-Specific Histone Demethylase 1A Inhibitor, in Relapsed or Refractory Acute Myeloid Leukemia
- PMID: 33052756
- PMCID: PMC7768337
- DOI: 10.1200/JCO.19.03250
First-in-Human Phase I Study of Iadademstat (ORY-1001): A First-in-Class Lysine-Specific Histone Demethylase 1A Inhibitor, in Relapsed or Refractory Acute Myeloid Leukemia
Abstract
Purpose: Iadademstat is a novel, highly potent, and selective inhibitor of LSD1 (KDM1A), with preclinical in vitro and in vivo antileukemic activity. This study aimed to determine safety and tolerability of iadademstat as monotherapy in patients with relapsed/refractory acute myeloid leukemia (R/R AML).
Methods: This phase I, nonrandomized, open-label, dose-escalation (DE), and extension-cohort (EC) trial included patients with R/R AML and evaluated the safety, pharmacokinetics (PK), pharmacodynamics (PD), and preliminary antileukemic activity of this orally bioavailable first-in-class lysine-specific demethylase 1 inhibitor.
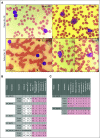
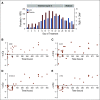
Results: Twenty-seven patients were treated with iadademstat on days 1 to 5 (5-220 µg/m2/d) of each week in 28-day cycles in a DE phase that resulted in a recommended dose of 140 µg/m2/d of iadademstat as a single agent. This dose was chosen to treat all patients (n = 14) in an EC enriched with patients with MLL/KMT2A-rearranged AML. Most adverse events (AEs) were as expected in R/R AML and included myelosuppression and nonhematologic AEs, such as infections, asthenia, mucositis, and diarrhea. PK data demonstrated a dose-dependent increase in plasma exposure, and PD data confirmed a potent time- and exposure-dependent induction of differentiation biomarkers. Reductions in blood and bone marrow blast percentages were observed, together with induction of blast cell differentiation, in particular, in patients with MLL translocations. One complete remission with incomplete count recovery was observed in the DE arm.
Conclusion: Iadademstat exhibits a good safety profile together with signs of clinical and biologic activity as a single agent in patients with R/R AML. A phase II trial of iadademstat in combination with azacitidine is ongoing (EudraCT No.: 2018-000482-36).
Figures


References
-
- Vardiman JW Thiele J Arber DA, et al. : The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood 114:937-951, 2009 - PubMed
-
- Döhner H Estey EH Amadori S, et al. : Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 115:453-474, 2010 - PubMed
-
- Döhner H, Weisdorf DJ, Bloomfield CD: Acute myeloid leukemia. N Engl J Med 373:1136-1152, 2015. - PubMed
-
- Burnett AK Milligan D Prentice AG, et al. : A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer 109:1114-1124, 2007 - PubMed

