ARTSCAN III: A Randomized Phase III Study Comparing Chemoradiotherapy With Cisplatin Versus Cetuximab in Patients With Locoregionally Advanced Head and Neck Squamous Cell Cancer
- PMID: 33052757
- PMCID: PMC7771720
- DOI: 10.1200/JCO.20.02072
ARTSCAN III: A Randomized Phase III Study Comparing Chemoradiotherapy With Cisplatin Versus Cetuximab in Patients With Locoregionally Advanced Head and Neck Squamous Cell Cancer
Abstract
Purpose: We performed an open-label randomized controlled phase III study comparing treatment outcome and toxicity between radiotherapy (RT) with concomitant cisplatin versus concomitant cetuximab in patients with locoregionally advanced head and neck squamous cell carcinoma (HNSCC; stage III-IV according to the Union for International Cancer Control TNM classification, 7th edition).
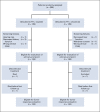
Materials and methods: Eligible patients were randomly assigned 1:1 to receive either intravenous cetuximab 400 mg/m2 1 week before start of RT followed by 250 mg/m2/wk, or weekly intravenous cisplatin 40 mg/m2, during RT. RT was conventionally fractionated. Patients with T3-T4 tumors underwent a second random assignment 1:1 between standard RT dose 68.0 Gy to the primary tumor or dose escalation to 73.1 Gy. Primary end point was overall survival (OS) evaluated using adjusted Cox regression analysis. Secondary end points were locoregional control, local control with dose-escalated RT, pattern of failure, and adverse effects.
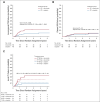
Results: Study inclusion was prematurely closed after an unplanned interim analysis when 298 patients had been randomly assigned. At 3 years, OS was 88% (95% CI, 83% to 94%) and 78% (95% CI, 71% to 85%) in the cisplatin and cetuximab groups, respectively (adjusted hazard ratio, 1.63; 95% CI, 0.93 to 2.86; P = .086). The cumulative incidence of locoregional failures at 3 years was 23% (95% CI, 16% to 31%) compared with 9% (95% CI, 4% to 14%) in the cetuximab versus the cisplatin group (Gray's test P = .0036). The cumulative incidence of distant failures did not differ between the treatment groups. Dose escalation in T3-T4 tumors did not increase local control.
Conclusion: Cetuximab is inferior to cisplatin regarding locoregional control for concomitant treatment with RT in patients with locoregionally advanced HNSCC. Additional studies are needed to identify possible subgroups that still may benefit from concomitant cetuximab treatment.
Trial registration: ClinicalTrials.gov NCT01969877.
Conflict of interest statement
No other potential conflicts of interest were reported.
Figures

Comment in
-
Treating Advanced Head and Neck Cancer When Cisplatin Is Not an Option.J Clin Oncol. 2021 Jan 1;39(1):7-12. doi: 10.1200/JCO.20.02720. Epub 2020 Dec 4. J Clin Oncol. 2021. PMID: 33275489
-
Something for Everyone From Low-Risk to High-Risk: 5 Recent Studies to Improve Treatment and Surveillance for All Patients With Squamous Cell Carcinoma of the Head and Neck.Int J Radiat Oncol Biol Phys. 2021 Sep 1;111(1):1-8. doi: 10.1016/j.ijrobp.2021.05.005. Int J Radiat Oncol Biol Phys. 2021. PMID: 34348102 No abstract available.
References
-
- Ferlay J, Ervik M, Lam F, et al (eds): Cancer Today (powered by GLOBOCAN 2018). Lyon, France, 2018.
-
- Sobin LH, Gospodarowicz MK, Wittekind C (eds): International Union Against Cancer (UICC): TNM Classification of Malignant Tumours (ed 7). Oxford, Wiley-Blackwell, 2009.
-
- Pignon JP le Maître A Maillard E, et al. : Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol 92:4-14, 2009 - PubMed
-
- Ho KF, Swindell R, Brammer CV: Dose intensity comparison between weekly and 3-weekly Cisplatin delivered concurrently with radical radiotherapy for head and neck cancer: A retrospective comparison from New Cross Hospital, Wolverhampton, UK. Acta Oncol 47:1513-1518, 2008 - PubMed
-
- Lau H Brar S Hao D, et al. : Concomitant low-dose cisplatin and three-dimensional conformal radiotherapy for locally advanced squamous cell carcinoma of the head and neck: Analysis of survival and toxicity. Head Neck 28:189-196, 2006 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

