Comparing DNA replication programs reveals large timing shifts at centromeres of endocycling cells in maize roots
- PMID: 33052904
- PMCID: PMC7588055
- DOI: 10.1371/journal.pgen.1008623
Comparing DNA replication programs reveals large timing shifts at centromeres of endocycling cells in maize roots
Abstract
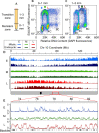
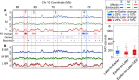
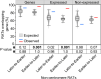
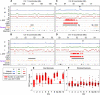
Plant cells undergo two types of cell cycles-the mitotic cycle in which DNA replication is coupled to mitosis, and the endocycle in which DNA replication occurs in the absence of cell division. To investigate DNA replication programs in these two types of cell cycles, we pulse labeled intact root tips of maize (Zea mays) with 5-ethynyl-2'-deoxyuridine (EdU) and used flow sorting of nuclei to examine DNA replication timing (RT) during the transition from a mitotic cycle to an endocycle. Comparison of the sequence-based RT profiles showed that most regions of the maize genome replicate at the same time during S phase in mitotic and endocycling cells, despite the need to replicate twice as much DNA in the endocycle and the fact that endocycling is typically associated with cell differentiation. However, regions collectively corresponding to 2% of the genome displayed significant changes in timing between the two types of cell cycles. The majority of these regions are small with a median size of 135 kb, shift to a later RT in the endocycle, and are enriched for genes expressed in the root tip. We found larger regions that shifted RT in centromeres of seven of the ten maize chromosomes. These regions covered the majority of the previously defined functional centromere, which ranged between 1 and 2 Mb in size in the reference genome. They replicate mainly during mid S phase in mitotic cells but primarily in late S phase of the endocycle. In contrast, the immediately adjacent pericentromere sequences are primarily late replicating in both cell cycles. Analysis of CENH3 enrichment levels in 8C vs 2C nuclei suggested that there is only a partial replacement of CENH3 nucleosomes after endocycle replication is complete. The shift to later replication of centromeres and possible reduction in CENH3 enrichment after endocycle replication is consistent with a hypothesis that centromeres are inactivated when their function is no longer needed.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Defining multiple, distinct, and shared spatiotemporal patterns of DNA replication and endoreduplication from 3D image analysis of developing maize (Zea mays L.) root tip nuclei.Plant Mol Biol. 2015 Nov;89(4-5):339-51. doi: 10.1007/s11103-015-0364-4. Epub 2015 Sep 22. Plant Mol Biol. 2015. PMID: 26394866 Free PMC article.
-
Genomic Analysis of the DNA Replication Timing Program during Mitotic S Phase in Maize (Zea mays) Root Tips.Plant Cell. 2017 Sep;29(9):2126-2149. doi: 10.1105/tpc.17.00037. Epub 2017 Aug 25. Plant Cell. 2017. PMID: 28842533 Free PMC article.
-
A maize root tip system to study DNA replication programmes in somatic and endocycling nuclei during plant development.J Exp Bot. 2014 Jun;65(10):2747-56. doi: 10.1093/jxb/ert470. Epub 2014 Jan 21. J Exp Bot. 2014. PMID: 24449386 Review.
-
Next-Generation Sequencing Enables Spatiotemporal Resolution of Human Centromere Replication Timing.Genes (Basel). 2019 Apr 2;10(4):269. doi: 10.3390/genes10040269. Genes (Basel). 2019. PMID: 30987063 Free PMC article.
-
[Regulation of DNA replication timing].Mol Biol (Mosk). 2013 Jan-Feb;47(1):12-37. doi: 10.1134/s0026893312060118. Mol Biol (Mosk). 2013. PMID: 23705493 Review. Russian.
Cited by
-
The structure, function, and evolution of plant centromeres.Genome Res. 2024 Mar 20;34(2):161-178. doi: 10.1101/gr.278409.123. Genome Res. 2024. PMID: 38485193 Free PMC article. Review.
-
A comparison of genomic methods to assess DNA replication timing.Sci Rep. 2025 May 22;15(1):17761. doi: 10.1038/s41598-025-02699-0. Sci Rep. 2025. PMID: 40404767 Free PMC article.
-
A Protocol for Genome-Wide Analysis of DNA Replication Timing in Intact Root Tips.Methods Mol Biol. 2022;2382:29-72. doi: 10.1007/978-1-0716-1744-1_3. Methods Mol Biol. 2022. PMID: 34705232
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

