Power-law population heterogeneity governs epidemic waves
- PMID: 33052918
- PMCID: PMC7556502
- DOI: 10.1371/journal.pone.0239678
Power-law population heterogeneity governs epidemic waves
Abstract
We generalize the Susceptible-Infected-Removed (SIR) model for epidemics to take into account generic effects of heterogeneity in the degree of susceptibility to infection in the population. We introduce a single new parameter corresponding to a power-law exponent of the susceptibility distribution at small susceptibilities. We find that for this class of distributions the gamma distribution is the attractor of the dynamics. This allows us to identify generic effects of population heterogeneity in a model as simple as the original SIR model which is contained as a limiting case. Because of this simplicity, numerical solutions can be generated easily and key properties of the epidemic wave can still be obtained exactly. In particular, we present exact expressions for the herd immunity level, the final size of the epidemic, as well as for the shape of the wave and for observables that can be quantified during an epidemic. In strongly heterogeneous populations, the herd immunity level can be much lower than in models with homogeneous populations as commonly used for example to discuss effects of mitigation. Using our model to analyze data for the SARS-CoV-2 epidemic in Germany shows that the reported time course is consistent with several scenarios characterized by different levels of immunity. These scenarios differ in population heterogeneity and in the time course of the infection rate, for example due to mitigation efforts or seasonality. Our analysis reveals that quantifying the effects of mitigation requires knowledge on the degree of heterogeneity in the population. Our work shows that key effects of population heterogeneity can be captured without increasing the complexity of the model. We show that information about population heterogeneity will be key to understand how far an epidemic has progressed and what can be expected for its future course.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

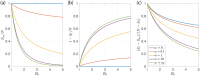

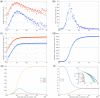
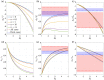

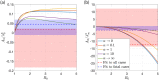

References
-
- Murray J. D. Mathematical Biology Interdisciplinary Applied Mathematics. Springer, New York, 3rd ed edition, 2002.
-
- Daley Daryl J and Gani J. M. Epidemic Modelling: An Introduction. Cambridge University Press, 1999.
-
- Hethcote Herbert W. The Mathematics of Infectious Diseases. SIAM Review, 42(4):599–653, January 2000. 10.1137/S0036144500371907 - DOI
-
- O. Diekmann, Hans Heesterbeek, and Tom Britton. Mathematical Tools for Understanding Infectious Diseases Dynamics. Princeton Series in Theoretical and Computational Biology. Princeton University Press, Princeton, 2013.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

