ECM deposition is driven by caveolin-1-dependent regulation of exosomal biogenesis and cargo sorting
- PMID: 33053168
- PMCID: PMC7551399
- DOI: 10.1083/jcb.202006178
ECM deposition is driven by caveolin-1-dependent regulation of exosomal biogenesis and cargo sorting
Abstract
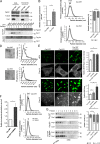
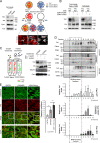
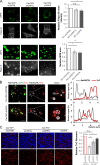
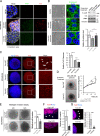
The composition and physical properties of the extracellular matrix (ECM) critically influence tumor progression, but the molecular mechanisms underlying ECM layering are poorly understood. Tumor-stroma interaction critically depends on cell communication mediated by exosomes, small vesicles generated within multivesicular bodies (MVBs). We show that caveolin-1 (Cav1) centrally regulates exosome biogenesis and exosomal protein cargo sorting through the control of cholesterol content at the endosomal compartment/MVBs. Quantitative proteomics profiling revealed that Cav1 is required for exosomal sorting of ECM protein cargo subsets, including Tenascin-C (TnC), and for fibroblast-derived exosomes to efficiently deposit ECM and promote tumor invasion. Cav1-driven exosomal ECM deposition not only promotes local stromal remodeling but also the generation of distant ECM-enriched stromal niches in vivo. Cav1 acts as a cholesterol rheostat in MVBs, determining sorting of ECM components into specific exosome pools and thus ECM deposition. This supports a model by which Cav1 is a central regulatory hub for tumor-stroma interactions through a novel exosome-dependent ECM deposition mechanism.
© 2020 Albacete-Albacete et al.
Figures








Similar articles
-
GPR143 controls ESCRT-dependent exosome biogenesis and promotes cancer metastasis.Dev Cell. 2023 Feb 27;58(4):320-334.e8. doi: 10.1016/j.devcel.2023.01.006. Epub 2023 Feb 16. Dev Cell. 2023. PMID: 36800996
-
PRNP/prion protein regulates the secretion of exosomes modulating CAV1/caveolin-1-suppressed autophagy.Autophagy. 2016 Nov;12(11):2113-2128. doi: 10.1080/15548627.2016.1226735. Epub 2016 Sep 14. Autophagy. 2016. PMID: 27629560 Free PMC article.
-
An inverted CAV1 (caveolin 1) topology defines novel autophagy-dependent exosome secretion from prostate cancer cells.Autophagy. 2021 Sep;17(9):2200-2216. doi: 10.1080/15548627.2020.1820787. Epub 2020 Sep 20. Autophagy. 2021. PMID: 32897127 Free PMC article.
-
The ins-and-outs of exosome biogenesis, secretion, and internalization.Trends Cell Biol. 2024 Feb;34(2):90-108. doi: 10.1016/j.tcb.2023.06.006. Epub 2023 Jul 26. Trends Cell Biol. 2024. PMID: 37507251 Free PMC article. Review.
-
Focus on the morphogenesis, fate and the role in tumor progression of multivesicular bodies.Cell Commun Signal. 2020 Aug 8;18(1):122. doi: 10.1186/s12964-020-00619-5. Cell Commun Signal. 2020. PMID: 32771015 Free PMC article. Review.
Cited by
-
Tumor-derived small extracellular vesicles in cancer invasion and metastasis: molecular mechanisms, and clinical significance.Mol Cancer. 2024 Jan 19;23(1):18. doi: 10.1186/s12943-024-01932-0. Mol Cancer. 2024. PMID: 38243280 Free PMC article. Review.
-
CD9 inhibition reveals a functional connection of extracellular vesicle secretion with mitophagy in melanoma cells.J Extracell Vesicles. 2021 May;10(7):e12082. doi: 10.1002/jev2.12082. Epub 2021 May 12. J Extracell Vesicles. 2021. PMID: 34012515 Free PMC article.
-
Stromal Fibroblasts Counteract the Caveolin-1-Dependent Radiation Response of LNCaP Prostate Carcinoma Cells.Front Oncol. 2022 Jan 26;12:802482. doi: 10.3389/fonc.2022.802482. eCollection 2022. Front Oncol. 2022. PMID: 35155239 Free PMC article.
-
Potential Applications and Functional Roles of Exosomes in Cardiometabolic Disease.Pharmaceutics. 2021 Dec 2;13(12):2056. doi: 10.3390/pharmaceutics13122056. Pharmaceutics. 2021. PMID: 34959338 Free PMC article. Review.
-
Biology of Tenascin C and its Role in Physiology and Pathology.Curr Med Chem. 2024;31(19):2706-2731. doi: 10.2174/0929867330666230404124229. Curr Med Chem. 2024. PMID: 37021423 Review.
References
-
- Augsten M., Sjöberg E., Frings O., Vorrink S.U., Frijhoff J., Olsson E., Borg Å., and Östman A.. 2014. Cancer-associated fibroblasts expressing CXCL14 rely upon NOS1-derived nitric oxide signaling for their tumor-supporting properties. Cancer Res. 74:2999–3010. 10.1158/0008-5472.CAN-13-2740 - DOI - PubMed
-
- Beacham D.A., Amatangelo M.D., and Cukierman E.. 2007. Preparation of extracellular matrices produced by cultured and primary fibroblasts. Curr. Protoc. Cell Biol. Chapter 10:Unit 10.9 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

