Stress-Induced Translation Inhibition through Rapid Displacement of Scanning Initiation Factors
- PMID: 33053322
- PMCID: PMC7657445
- DOI: 10.1016/j.molcel.2020.09.021
Stress-Induced Translation Inhibition through Rapid Displacement of Scanning Initiation Factors
Abstract
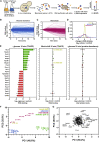
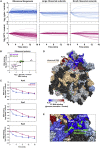
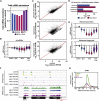
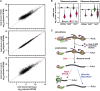
Cellular responses to environmental stress are frequently mediated by RNA-binding proteins (RBPs). Here, we examined global RBP dynamics in Saccharomyces cerevisiae in response to glucose starvation and heat shock. Each stress induced rapid remodeling of the RNA-protein interactome without corresponding changes in RBP abundance. Consistent with general translation shutdown, ribosomal proteins contacting the mRNA showed decreased RNA association. Among translation components, RNA association was most reduced for initiation factors involved in 40S scanning (eukaryotic initiation factor 4A [eIF4A], eIF4B, and Ded1), indicating a common mechanism of translational repression. In unstressed cells, eIF4A, eIF4B, and Ded1 primarily targeted the 5' ends of mRNAs. Following glucose withdrawal, 5' binding was abolished within 30 s, explaining the rapid translation shutdown, but mRNAs remained stable. Heat shock induced progressive loss of 5' RNA binding by initiation factors over ∼16 min and provoked mRNA degradation, particularly for translation-related factors, mediated by Xrn1. Taken together, these results reveal mechanisms underlying translational control of gene expression during stress.
Keywords: RNA-binding sites; UV crosslinking; mass spectrometry; protein-RNA interaction; stress responses; yeast.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

