A Comprehensive Subcellular Atlas of the Toxoplasma Proteome via hyperLOPIT Provides Spatial Context for Protein Functions
- PMID: 33053376
- PMCID: PMC7670262
- DOI: 10.1016/j.chom.2020.09.011
A Comprehensive Subcellular Atlas of the Toxoplasma Proteome via hyperLOPIT Provides Spatial Context for Protein Functions
Abstract
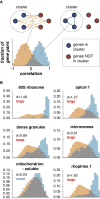
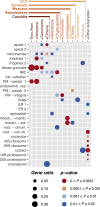
Apicomplexan parasites cause major human disease and food insecurity. They owe their considerable success to highly specialized cell compartments and structures. These adaptations drive their recognition, nondestructive penetration, and elaborate reengineering of the host's cells to promote their growth, dissemination, and the countering of host defenses. The evolution of unique apicomplexan cellular compartments is concomitant with vast proteomic novelty. Consequently, half of apicomplexan proteins are unique and uncharacterized. Here, we determine the steady-state subcellular location of thousands of proteins simultaneously within the globally prevalent apicomplexan parasite Toxoplasma gondii. This provides unprecedented comprehensive molecular definition of these unicellular eukaryotes and their specialized compartments, and these data reveal the spatial organizations of protein expression and function, adaptation to hosts, and the underlying evolutionary trajectories of these pathogens.
Keywords: apicomplexa; evolution; host-pathogen interaction; invasion; organelle; parasitism; plasmodium; proteomics; subcellular; toxoplasma.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures






References
-
- Almagro Armenteros J.J., Tsirigos K.D., Sønderby C.K., Petersen T.N., Winther O., Brunak S., von Heijne G., Nielsen H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019;37:420–423. - PubMed
-
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B. 1995;57:289–300.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

