The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier
- PMID: 33053430
- PMCID: PMC7547916
- DOI: 10.1016/j.nbd.2020.105131
The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier
Abstract
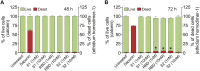
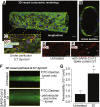
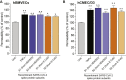
As researchers across the globe have focused their attention on understanding SARS-CoV-2, the picture that is emerging is that of a virus that has serious effects on the vasculature in multiple organ systems including the cerebral vasculature. Observed effects on the central nervous system include neurological symptoms (headache, nausea, dizziness), fatal microclot formation and in rare cases encephalitis. However, our understanding of how the virus causes these mild to severe neurological symptoms and how the cerebral vasculature is impacted remains unclear. Thus, the results presented in this report explored whether deleterious outcomes from the SARS-CoV-2 viral spike protein on primary human brain microvascular endothelial cells (hBMVECs) could be observed. The spike protein, which plays a key role in receptor recognition, is formed by the S1 subunit containing a receptor binding domain (RBD) and the S2 subunit. First, using postmortem brain tissue, we show that the angiotensin converting enzyme 2 or ACE2 (a known binding target for the SARS-CoV-2 spike protein), is ubiquitously expressed throughout various vessel calibers in the frontal cortex. Moreover, ACE2 expression was upregulated in cases of hypertension and dementia. ACE2 was also detectable in primary hBMVECs maintained under cell culture conditions. Analysis of cell viability revealed that neither the S1, S2 or a truncated form of the S1 containing only the RBD had minimal effects on hBMVEC viability within a 48 h exposure window. Introduction of spike proteins to invitro models of the blood-brain barrier (BBB) showed significant changes to barrier properties. Key to our findings is the demonstration that S1 promotes loss of barrier integrity in an advanced 3D microfluidic model of the human BBB, a platform that more closely resembles the physiological conditions at this CNS interface. Evidence provided suggests that the SARS-CoV-2 spike proteins trigger a pro-inflammatory response on brain endothelial cells that may contribute to an altered state of BBB function. Together, these results are the first to show the direct impact that the SARS-CoV-2 spike protein could have on brain endothelial cells; thereby offering a plausible explanation for the neurological consequences seen in COVID-19 patients.
Keywords: Blood-brain barrier; COVID-19; Cerebral vascular biology; Microfluidic chip; Neuroinflammation; SARS-CoV-2; Tissue engineering.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures




Update of
-
The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in vitro models of the human blood-brain barrier.bioRxiv [Preprint]. 2020 Jun 15:2020.06.15.150912. doi: 10.1101/2020.06.15.150912. bioRxiv. 2020. Update in: Neurobiol Dis. 2020 Dec;146:105131. doi: 10.1016/j.nbd.2020.105131. PMID: 32587958 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

