Clinical Application of Oncolytic Viruses: A Systematic Review
- PMID: 33053757
- PMCID: PMC7589713
- DOI: 10.3390/ijms21207505
Clinical Application of Oncolytic Viruses: A Systematic Review
Abstract
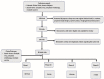
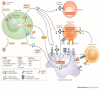
Leveraging the immune system to thwart cancer is not a novel strategy and has been explored via cancer vaccines and use of immunomodulators like interferons. However, it was not until the introduction of immune checkpoint inhibitors that we realized the true potential of immunotherapy in combating cancer. Oncolytic viruses are one such immunotherapeutic tool that is currently being explored in cancer therapeutics. We present the most comprehensive systematic review of all oncolytic viruses in Phase 1, 2, and 3 clinical trials published to date. We performed a systematic review of all published clinical trials indexed in PubMed that utilized oncolytic viruses. Trials were reviewed for type of oncolytic virus used, method of administration, study design, disease type, primary outcome, and relevant adverse effects. A total of 120 trials were found; 86 trials were available for our review. Included were 60 phase I trials, five phase I/II combination trials, 19 phase II trials, and two phase III clinical trials. Oncolytic viruses are feverously being evaluated in oncology with over 30 different types of oncolytic viruses being explored either as a single agent or in combination with other antitumor agents. To date, only one oncolytic virus therapy has received an FDA approval but advances in bioengineering techniques and our understanding of immunomodulation to heighten oncolytic virus replication and improve tumor kill raises optimism for its future drug development.
Keywords: clinical trials; immunotherapy; oncolytic viruses.
Conflict of interest statement
Mary Cook has no conflicts to report. Aman Chauhan has received research support from BMS, Clovis, Entrinsic Health Solution, EMD Serono, and Lexicon Pharmaceuticals.
Figures
References
-
- Amgen FDA Approves IMLYGICTM (Talimogene Laherparepvic) as First Oncolytic Viral Therapy in the US. [(accessed on 5 January 2020)];2018 Available online: https://www.prnewswire.com/news-releases/fda-approves-imlygic-talimogene....
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources