Functions of Osteocalcin in Bone, Pancreas, Testis, and Muscle
- PMID: 33053789
- PMCID: PMC7589887
- DOI: 10.3390/ijms21207513
Functions of Osteocalcin in Bone, Pancreas, Testis, and Muscle
Abstract
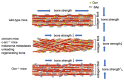
Osteocalcin (Ocn), which is specifically produced by osteoblasts, and is the most abundant non-collagenous protein in bone, was demonstrated to inhibit bone formation and function as a hormone, which regulates glucose metabolism in the pancreas, testosterone synthesis in the testis, and muscle mass, based on the phenotype of Ocn-/- mice by Karsenty's group. Recently, Ocn-/- mice were newly generated by two groups independently. Bone strength is determined by bone quantity and quality. The new Ocn-/- mice revealed that Ocn is not involved in the regulation of bone formation and bone quantity, but that Ocn regulates bone quality by aligning biological apatite (BAp) parallel to the collagen fibrils. Moreover, glucose metabolism, testosterone synthesis and spermatogenesis, and muscle mass were normal in the new Ocn-/- mice. Thus, the function of Ocn is the adjustment of growth orientation of BAp parallel to the collagen fibrils, which is important for bone strength to the loading direction of the long bone. However, Ocn does not play a role as a hormone in the pancreas, testis, and muscle. Clinically, serum Ocn is a marker for bone formation, and exercise increases bone formation and improves glucose metabolism, making a connection between Ocn and glucose metabolism.
Keywords: apatite crystal; bone formation; bone strength; collagen; glucose metabolism; muscle; osteocalcin; testosterone.
Conflict of interest statement
The author declares no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

