Roles of the complement system in alcohol-induced liver disease
- PMID: 33053939
- PMCID: PMC7641541
- DOI: 10.3350/cmh.2020.0094
Roles of the complement system in alcohol-induced liver disease
Abstract
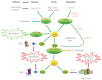
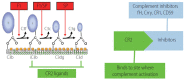
Alcohol-induced liver disease (ALD) is a complex disorder, with a disease spectrum ranging from steatosis to steatohepatitis, cirrhosis, and hepatocellular carcinoma. Although the pathogenesis of ALD is incompletely understood and currently no effective drugs are available for ALD, several lines of evidence suggest that complement activation and oxidative stress play crucial roles in the pathogenesis of ALD. Complement activation can regulate the production of ROS and influence oxidative stress in ALD. Precise regulation of the complement system in ALD may be a rational and novel avenue to postpone and even reverse the progression of disease and simultaneously promote the repair of liver injury. In this mini-review, we briefly summarize the recent research progress, especially focusing on the role of complement and oxidative stress-induced transfer RNA-derived fragments, which might help us to better understand the pathogenesis of ALD and provide aid in the development of novel therapeutic strategies for ALD.
Keywords: Alcoholic; Complement system proteins; Liver Diseases; Molecular targeted therapy.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures

References
-
- Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol. 2004;41:1089–1098. - PubMed
-
- Fey G, Colten HR. Biosynthesis of complement components. Fed Proc. 1981;40:2099–2104. - PubMed
-
- Wlazlo N, van Greevenbroek MM, Ferreira I, Jansen EH, Feskens EJ, van der Kallen CJ, et al. Activated complement factor 3 is associated with liver fat and liver enzymes: the CODAM study. Eur J Clin Invest. 2013;43:679–688. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

