Oxidative stress and glutamate excretion in alcoholic steatosis: Metabolic synapse between hepatocyte and stellate cell
- PMID: 33053940
- PMCID: PMC7641576
- DOI: 10.3350/cmh.2020.0152
Oxidative stress and glutamate excretion in alcoholic steatosis: Metabolic synapse between hepatocyte and stellate cell
Abstract
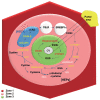
Chronic alcohol consumption induces the development of alcoholic steatosis in the liver, which is one of the most widespread liver diseases worldwide. During general alcohol metabolism, hepatocytes generate mitochondria- and cytochrome P450 2E1 (CYP2E1)-mediated reactive oxygen species (ROS) whose accumulation elicits activation of the hepatic anti-oxidant system, including glutathione (GSH). However, chronic alcohol consumption decreases GSH generation through cysteine deficiency by suppressing the methionine cycle and trans-sulfuration system, whereas it turns on an alternative defense pathway, such as the xCT transporter, to compensate for GSH shortage. The xCT transporter mediates the uptake of cystine coupled to the efflux of glutamate, leading to an increase in blood glutamate. In response to the elevated glutamate in the liver, the expression of metabotropic glutamate receptor 5 (mGluR5) is up-regulated in hepatic stellate cells (HSCs) along with enhanced production of 2-arachidonoylglycerol, which in turn stimulates cannabinoid receptor 1 (CB1R) on neighboring hepatocytes to increase de novo lipogenesis. On the other hand, blockade of mGluR5 and CB1R attenuates alcoholic steatosis. Interestingly, although the increased expression of CYP2E1-mediated xCT and ROS generation are mainly observed at the perivenous area (zone 3), fat accumulation is mostly detected at hepatic zone 2. To resolve this discrepancy, this review summarizes recent advances on glutamate/mGluR5-derived alcoholic steatosis and zone-dependently different responses to alcohol intake. In addition, the bidirectional loop pathway and its unique metabolic synapse between hepatocytes and HSCs are discussed.
Keywords: Alcoholic liver disease; Aldehyde dehydrogenase; Diacylglycerol lipase; Endocannabinoids; Homocysteine.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

