Why is it critical to achieve a deep molecular response in chronic myeloid leukemia?
- PMID: 33054104
- PMCID: PMC7716360
- DOI: 10.3324/haematol.2019.240739
Why is it critical to achieve a deep molecular response in chronic myeloid leukemia?
Abstract
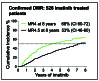
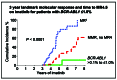
The primary goal of tyrosine kinase inhibitor (TKI) therapy for patients with chronic myeloid leukemia is survival, which is achieved by the vast majority of patients. The initial response to therapy provides a sensitive measure of future clinical outcome. Measurement of BCR-ABL1 transcript levels using real-time quantitative polymerase chain reaction standardized to the international reporting scale is now the principal recommended monitoring strategy. The method is used to assess early milestone responses and provides a guide for therapeutic intervention. When patients successfully traverse the critical first 12 months of TKI therapy, most will head towards another milestone response, deep molecular response (DMR, BCR-ABL1 ≤0.01%). DMR is essential for patients aiming to achieve treatment-free remission and a prerequisite for a trial of TKI discontinuation. The success of discontinuation trials has led to new treatment strategies in order for more patients to reach this milestone response. DMR has been incorporated into endpoints of clinical trials and is considered by some expert groups as the optimal treatment response. But is DMR a stable response and does it provide the ultimate protection against TKI resistance and death? Do we need to increase the sensitivity of detection of BCR-ABL1 to better identify the patients who would likely remain in treatment-free remission after TKI discontinuation? Is it necessary to switch current TKI therapy to a more potent inhibitor if the goal is to achieve DMR? These are issues that I will explore in this review.
Figures

References
-
- O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and lowdose cytarabine for newly diagnosed chronic- phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994-1004. - PubMed
-
- Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349(15):1423-1432. - PubMed
-
- Hughes TP, Branford S. Monitoring disease response to tyrosine kinase inhibitor therapy in CML. Hematology Am Soc Hematol Educ Program. 2009;2009(1):477-487. - PubMed
-
- Lipton JH, Chuah C, Guerci-Bresler A, et al. Ponatinib versus imatinib for newly diagnosed chronic myeloid leukaemia: an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(5):612-621. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

