Pediatric acute lymphoblastic leukemia
- PMID: 33054110
- PMCID: PMC7604619
- DOI: 10.3324/haematol.2020.247031
Pediatric acute lymphoblastic leukemia
Abstract
The last decade has witnessed great advances in our understanding of the genetic and biological basis of childhood acute lymphoblastic leukemia (ALL), the development of experimental models to probe mechanisms and evaluate new therapies, and the development of more efficacious treatment stratification. Genomic analyses have revolutionized our understanding of the molecular taxonomy of ALL, and these advances have led the push to implement genome and transcriptome characterization in the clinical management of ALL to facilitate more accurate risk-stratification and, in some cases, targeted therapy. Although mutation- or pathway-directed targeted therapy (e.g., using tyrosine kinase inhibitors to treat Philadelphia chromosome [Ph]-positive and Phlike B-cell-ALL) is currently available for only a minority of children with ALL, many of the newly identified molecular alterations have led to the exploration of approaches targeting deregulated cell pathways. The efficacy of cellular or humoral immunotherapy has been demonstrated with the success of chimeric antigen receptor T-cell therapy and the bispecific engager blinatumomab in treating advanced disease. This review describes key advances in our understanding of the biology of ALL and optimal approaches to risk-stratification and therapy, and it suggests key areas for basic and clinical research.
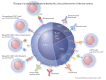
Figures
References
-
- Moricke A, Zimmermann M, Valsecchi MG, et al. Dexamethasone versus prednisone in induction treatment of pediatric ALL: results of the randomized trial AIEOP-BFM ALL 2000. Blood. 2016;127(17):2101-2112. - PubMed
-
- Place AE, Stevenson KE, Vrooman LM, et al. Intravenous pegylated asparaginase versus intramuscular native Escherichia coli Lasparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05-001): a randomised, open-label phase 3 trial. Lancet Oncol. 2015;16(16):1677-1690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

