Butyrate mediates anti-inflammatory effects of Faecalibacterium prausnitzii in intestinal epithelial cells through Dact3
- PMID: 33054518
- PMCID: PMC7567499
- DOI: 10.1080/19490976.2020.1826748
Butyrate mediates anti-inflammatory effects of Faecalibacterium prausnitzii in intestinal epithelial cells through Dact3
Abstract
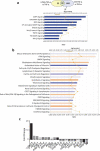
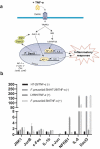
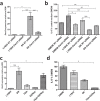
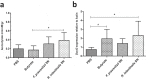
The commensal bacterium Faecalibacterium prausnitzii plays a key role in inflammatory bowel disease (IBD) pathogenesis and serves as a general health biomarker in humans. However, the host molecular mechanisms that underlie its anti-inflammatory effects remain unknown. In this study we performed a transcriptomic approach on human intestinal epithelial cells (HT-29) stimulated with TNF-α and exposed to F. prausnitzii culture supernatant (SN) in order to determine the impact of this commensal bacterium on intestinal epithelial cells. Moreover, modulation of the most upregulated gene after F. prausnitzii SN contact was validated both in vitro and in vivo. Our results showed that F. prausnitzii SN upregulates the expression of Dact3, a gene linked to the Wnt/JNK pathway. Interestingly, when we silenced Dact3 expression, the effect of F. prausnitzii SN was lost. Butyrate was identified as the F. prausnitzii effector responsible for Dact3 modulation. Dact3 upregulation was also validated in vivo in both healthy and inflamed mice treated with either F. prausnitzii SN or the live bacteria, respectively. Finally, we demonstrated by colon transcriptomics that gut microbiota directly influences Dact3 expression. This study provides new clues about the host molecular mechanisms involved in the anti-inflammatory effects of the beneficial commensal bacterium F. prausnitzii.
Keywords: Faecalibacterium prausnitzii; Commensal bacteria; inflammatory bowel disease; signaling pathway; transcriptomic analysis.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
