Mechanisms of Drug-Induced Tolerance in Mycobacterium tuberculosis
- PMID: 33055230
- PMCID: PMC7566895
- DOI: 10.1128/CMR.00141-20
Mechanisms of Drug-Induced Tolerance in Mycobacterium tuberculosis
Abstract
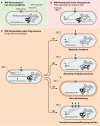
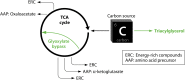
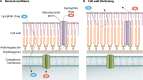
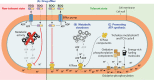
Successful treatment of tuberculosis (TB) can be hampered by Mycobacterium tuberculosis populations that are temporarily able to survive antibiotic pressure in the absence of drug resistance-conferring mutations, a phenomenon termed drug tolerance. We summarize findings on M. tuberculosis tolerance published in the past 20 years. Key M. tuberculosis responses to drug pressure are reduced growth rates, metabolic shifting, and the promotion of efflux pump activity. Metabolic shifts upon drug pressure mainly occur in M. tuberculosis's lipid metabolism and redox homeostasis, with reduced tricarboxylic acid cycle activity in favor of lipid anabolism. Increased lipid anabolism plays a role in cell wall thickening, which reduces sensitivity to most TB drugs. In addition to these general mechanisms, drug-specific mechanisms have been described. Upon isoniazid exposure, M. tuberculosis reprograms several pathways associated with mycolic acid biosynthesis. Upon rifampicin exposure, M. tuberculosis upregulates the expression of its drug target rpoB Upon bedaquiline exposure, ATP synthesis is stimulated, and the transcription factors Rv0324 and Rv0880 are activated. A better understanding of M. tuberculosis's responses to drug pressure will be important for the development of novel agents that prevent the development of drug tolerance following treatment initiation. Such agents could then contribute to novel TB treatment-shortening strategies.
Keywords: Mycobacterium tuberculosis; WhiB regulon; drug tolerance; efflux pumps; lipid metabolism; metabolic shifting; metabolic slowdown; mycobacterial cell wall; redox homeostasis; sigma factors.
Copyright © 2020 American Society for Microbiology.
Figures

References
-
- WHO. 2019. Global tuberculosis report 2019. WHO, Geneva, Switzerland: https://www.who.int/tb/publications/global_report/en/.
-
- WHO. 2018. Global tuberculosis report 2018. WHO, Geneva, Switzerland: https://www.who.int/tb/publications/global_report/en/.
-
- Yihunie Akalu T, Muchie KF, Alemu Gelaye K. 2018. Time to sputum culture conversion and its determinants among multi-drug resistant tuberculosis patients at public hospitals of the Amhara Regional State: a multicenter retrospective follow up study. PLoS One 13:e0199320. doi:10.1371/journal.pone.0199320. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

