Quadruple mutation GCAC1809-1812TTCT acts as a biomarker in healthy European HBV carriers
- PMID: 33055418
- PMCID: PMC7710305
- DOI: 10.1172/jci.insight.135833
Quadruple mutation GCAC1809-1812TTCT acts as a biomarker in healthy European HBV carriers
Abstract
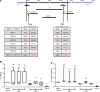
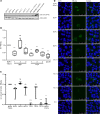
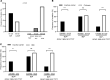
Many mutation analyses of the HBV genome have been performed in the search for new prognostic markers. However, the Kozak sequence preceding precore was covered only infrequently in these analyses. In this study, the HBV core promoter/precore region was sequenced in serum samples from European inactive HBV carriers. Quadruple mutation GCAC1809-1812TTCT was found with a high prevalence of 42% in the Kozak sequence preceding precore among all HBV genotypes. GCAC1809-1812TTCT was strongly associated with coexistence of basal core promoter (BCP) double mutation A1762T/G1764A and lower HBV DNA levels. In vitro GCAC1809-1812TTCT lead to drastically diminished synthesis of pregenomic RNA (pgRNA), precore mRNA, core, HBsAg, and HBeAg. Calculation of the pgRNA secondary structure suggests a destabilization of the pgRNA structure by A1762T/G1764A that was compensated by GCAC1809-1812TTCT. In 125 patients with HBV-related cirrhosis, GCAC1809-1812TTCT was not detected. While a strong association of GCAC1809-1812TTCT with inactive carrier status was observed, BCP double mutation was strongly correlated with cirrhosis, but this was only observed in absence of GCAC1809-1812TTCT. In conclusion, our data reveal that GCAC1809-1812TTCT is highly prevalent in inactive carriers and acts as a compensatory mutation for BCP double mutation. GCAC1809-1812TTCT seems to be a biomarker of good prognosis in HBV infection.
Keywords: Clinical practice; Hepatology; Molecular biology; Virology.
Conflict of interest statement
Figures



References
-
- [No authors listed]. World Health Organization. Hepatitis B fact sheet. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b Updated July 27, 2020. Accessed October 12, 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

