Long non-coding RNA CASC9 promotes gefitinib resistance in NSCLC by epigenetic repression of DUSP1
- PMID: 33056982
- PMCID: PMC7560854
- DOI: 10.1038/s41419-020-03047-y
Long non-coding RNA CASC9 promotes gefitinib resistance in NSCLC by epigenetic repression of DUSP1
Abstract
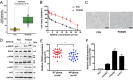
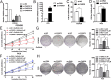
Resistance to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs), such as gefitinib, has greatly affected clinical outcomes in non-small cell lung cancer (NSCLC) patients. The long noncoding RNAs (lncRNAs) are known to regulate tumorigenesis and cancer progression, but their contributions to NSCLC gefitinib resistance remain poorly understood. In this study, by analyzing the differentially expressed lncRNAs in gefitinib-resistant cells and gefitinib-sensitive cells in the National Institute of Health GEO dataset, we found that lncRNA CASC9 expression was upregulated, and this was also verified in resistant tissues. Gain and loss of function studies showed that CASC9 inhibition restored gefitinib sensitivity both in vitro and in vivo, whereas CASC9 overexpression promoted gefitinib resistance. Mechanistically, CASC9 repressed the tumor suppressor DUSP1 by recruiting histone methyltransferase EZH2, thereby increasing the resistance to gefitinib. Furthermore, ectopic expression of DUSP1 increased gefitinib sensitivity by inactivating the ERK pathway. Our results highlight the essential role of CASC9 in gefitinib resistance, suggesting that the CASC9/EZH2/DUSP1 axis might be a novel target for overcoming EGFR-TKI resistance in NSCLC.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

