Impact of angiotensin receptor blocker product recalls on antihypertensive prescribing in Germany
- PMID: 33057175
- PMCID: PMC8502678
- DOI: 10.1038/s41371-020-00425-z
Impact of angiotensin receptor blocker product recalls on antihypertensive prescribing in Germany
Abstract
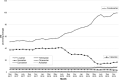
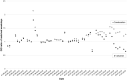
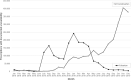
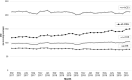
In Germany, ~8 million patients take angiotensin receptor blockers (ARBs) and 2.25 million of them valsartan. In 2018, contamination of generic ARBs with probable carcinogenic nitrosamines resulted in more than 30 recalls. The impact of such a huge recall has never been explored in Europe. We analyzed the utilization of valsartan, all ARBs, and other alternative antihypertensive drugs in Germany. We used our database of anonymized dispensing data from >80% of community pharmacies at the expense of the statutory health insurance (SHI) funds from January 2017 to December 2019. We analyzed 290.8 million prescriptions, including all oral mono- and fixed-dose combinations of ARBs and plausible alternatives, i.e. ACE inhibitors (ACEi), beta-blockers (BB), and calcium channel blockers (CCB). Utilization was calculated by defined daily doses per 1000 SHI-insured persons per day (DID). Valsartan use decreased substantially after the recalls in July 2018 from 39.0 to 14.2 DID (-64%) in the second quarter of 2019 and to 16.9 DID (-57%) in the fourth quarter of 2019. Simultaneously, the use of alternative ARBs increased from 77.7 DID in the second quarter of 2018 to 121.9 DID (+57%) in the fourth quarter of 2019, mainly due to an increase of candesartan dispensing to 99.8 DID (+73%). There were no changes in the utilization of ACEi, BB, or CCB. The majority of recalled generic valsartan products were replaced by other ARBs, predominantly candesartan, despite documented drug shortages. In contrast to previous safety warnings/recalls, our data do not suggest an under-prescription of antihypertensives during this period.
© 2020. The Author(s).
Conflict of interest statement
UR has received scientific support and speaker honoraria from Bayer, Berlin Chemie, and Bristol Myers Squibb, all outside the submitted work. FM is supported by Deutsche Gesellschaft für Kardiologie (DGK) and has received scientific support and speaker honoraria from Bayer, Boehringer Ingelheim, Medtronic, and ReCor Medical, all outside the submitted work. FM and MB are supported by the Deutsche Forschungsgemeinschaft (SFB TRR219). UL has received speaker honoraria from Bayer, Boehringer Ingelheim, Novartis and Servier, all outside the submitted work. MS has received speaker honoraria from Novartis and Sanofi, all outside the submitted work. All other authors declare no conflict of interest.
Figures
References
-
- Bundesministerium für Gesundheit. Verunreinigungen von Arzneimitteln mit Valsartan. Berlin: Bundesministerium für Gesundheit; 2018. https://kleineanfragen.de/bundestag/19/4073.
-
- BfArM. Valsartan: chargenbezogener Rückruf valsartanhaltiger Arzneimittel, deren Wirkstoff von dem chinesischen Hersteller Zhejiang Huahai Pharmaceutical produziert wurde: Pressemitteilung 5/2018. Bonn, DE: BfArM; 2018. https://www.bfarm.de/SharedDocs/Pressemitteilungen/DE/2018/pm5-2018.html.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

