The cellular basis of distinct thirst modalities
- PMID: 33057193
- PMCID: PMC7718410
- DOI: 10.1038/s41586-020-2821-8
The cellular basis of distinct thirst modalities
Abstract
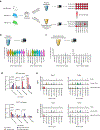
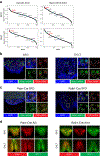
Fluid intake is an essential innate behaviour that is mainly caused by two distinct types of thirst1-3. Increased blood osmolality induces osmotic thirst that drives animals to consume pure water. Conversely, the loss of body fluid induces hypovolaemic thirst, in which animals seek both water and minerals (salts) to recover blood volume. Circumventricular organs in the lamina terminalis are critical sites for sensing both types of thirst-inducing stimulus4-6. However, how different thirst modalities are encoded in the brain remains unknown. Here we employed stimulus-to-cell-type mapping using single-cell RNA sequencing to identify the cellular substrates that underlie distinct types of thirst. These studies revealed diverse types of excitatory and inhibitory neuron in each circumventricular organ structure. We show that unique combinations of these neuron types are activated under osmotic and hypovolaemic stresses. These results elucidate the cellular logic that underlies distinct thirst modalities. Furthermore, optogenetic gain of function in thirst-modality-specific cell types recapitulated water-specific and non-specific fluid appetite caused by the two distinct dipsogenic stimuli. Together, these results show that thirst is a multimodal physiological state, and that different thirst states are mediated by specific neuron types in the mammalian brain.
Conflict of interest statement
The authors declare no competing financial interests.
Figures