Capillary cell-type specialization in the alveolus
- PMID: 33057196
- PMCID: PMC7721049
- DOI: 10.1038/s41586-020-2822-7
Capillary cell-type specialization in the alveolus
Abstract
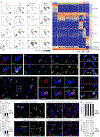
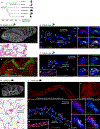
In the mammalian lung, an apparently homogenous mesh of capillary vessels surrounds each alveolus, forming the vast respiratory surface across which oxygen transfers to the blood1. Here we use single-cell analysis to elucidate the cell types, development, renewal and evolution of the alveolar capillary endothelium. We show that alveolar capillaries are mosaics; similar to the epithelium that lines the alveolus, the alveolar endothelium is made up of two intermingled cell types, with complex 'Swiss-cheese'-like morphologies and distinct functions. The first cell type, which we term the 'aerocyte', is specialized for gas exchange and the trafficking of leukocytes, and is unique to the lung. The other cell type, termed gCap ('general' capillary), is specialized to regulate vasomotor tone, and functions as a stem/progenitor cell in capillary homeostasis and repair. The two cell types develop from bipotent progenitors, mature gradually and are affected differently in disease and during ageing. This cell-type specialization is conserved between mouse and human lungs but is not found in alligator or turtle lungs, suggesting it arose during the evolution of the mammalian lung. The discovery of cell type specialization in alveolar capillaries transforms our understanding of the structure, function, regulation and maintenance of the air-blood barrier and gas exchange in health, disease and evolution.
Conflict of interest statement
Figures













References
-
- Malpighi M “Dissertationes Epistolicæ de Pulmonibus” in Opera Omnia, 320–332 (Pieter van der Aa, 1687). https://www.biodiversitylibrary.org/bibliography/566#.
-
- Weibel ER Morphological basis of alveolar-capillary gas exchange. Physiol Rev 53, 419–495 (1973). - PubMed
-
- Bertalanffy FD & Leblond CP Structure of respiratory tissue. Lancet 269, 1365–1368 (1955). - PubMed
Online-only References
-
- Tian X, Hu T, Zhang H, He L, Huang X, Liu Q, Yu W, He L, Yang Z, Zhang Z, Zhong TP, Yang X, Yang Z, Yan Y, Baldini A, Sun Y, Lu J, Schwartz RJ, Evans SM, Gittenberger-de Groot AC, Red-Horse K & Zhou B Subepicardial endothelial cells invade the embryonic ventricle wall to form coronary arteries. Cell Res 23, 1075–1090 (2013). - PMC - PubMed
-
- Chen HI, Sharma B, Akerberg BN, Numi HJ, Kivela R, Saharinen P, Aghajanian H, McKay AS, Bogard PE, Chang AH, Jacobs AH, Epstein JA, Stankunas K, Alitalo K & Red-Horse K The sinus venosus contributes to coronary vasculature through VEGFC-stimulated angiogenesis. Development 141, 4500–4512 (2014). - PMC - PubMed
-
- Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Luthi U, Barberis A, Benjamin LE, Makinen T, Nobes CD & Adams RH Ephrin-B2 controls Vegf-induced angiogenesis and lymphangiogenesis. Nature 465, 483–486 (2010). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

