SOX2 regulates homeostasis of taste bud cells and lingual epithelial cells in posterior tongue
- PMID: 33057384
- PMCID: PMC7561181
- DOI: 10.1371/journal.pone.0240848
SOX2 regulates homeostasis of taste bud cells and lingual epithelial cells in posterior tongue
Abstract
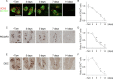
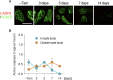
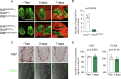
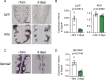
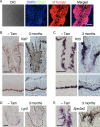
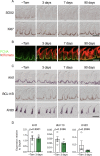
Taste bud cells arise from local epithelial stem cells in the oral cavity and are continuously replaced by newborn cells throughout an animal's life. However, little is known about the molecular and cellular mechanisms of taste cell turnover. Recently, it has been demonstrated that SOX2, a transcription factor expressed in epithelial stem/progenitor cells of the oral cavity, regulates turnover of anterior tongue epithelium including gustatory and non-gustatory papillae. Yet, the role of SOX2 in regulating taste cell turnover in the posterior tongue is unclear. Prompted by the fact that there are regional differences in the cellular and molecular composition of taste buds and stem/progenitor cells in the anterior and posterior portions of tongue, which are derived from distinct embryonic origins, we set out to determine the role of SOX2 in epithelial tissue homeostasis in the posterior tongue. Here we report the differential requirement of SOX2 in the stem/progenitor cells for the normal turnover of lingual epithelial cells in the posterior tongue. Sox2 deletion in the stem/progenitor cells neither induced active caspase 3-mediated apoptotic cell death nor altered stem/progenitor cell population in the posterior tongue. Nevertheless, morphology and molecular feature of non-gustatory epithelial cells were impaired in the circumvallate papilla but not in the filiform papillae. Remarkably, taste buds became thinner, collapsed, and undetectable over time. Lineage tracing of Sox2-deleted stem/progenitor cells demonstrated an almost complete lack of newly generated basal precursor cells in the taste buds, suggesting mechanistically that Sox2 is involved in determining stem/progenitor cells to differentiate to gustatory lineage cells. Together, these results demonstrate that SOX2 plays key roles in regulating epithelial tissue homeostasis in the posterior tongue, similar but not identical to its function in the anterior tongue.
Conflict of interest statement
NO authors have competing interests.
Figures

Similar articles
-
Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate.Stem Cells. 2009 Feb;27(2):442-50. doi: 10.1634/stemcells.2008-0611. Stem Cells. 2009. PMID: 19038788 Free PMC article.
-
Genetic Lineage Tracing in Taste Tissues Using Sox2-CreERT2 Strain.Chem Senses. 2017 Sep 1;42(7):547-552. doi: 10.1093/chemse/bjx032. Chem Senses. 2017. PMID: 28595328 Free PMC article.
-
Early taste buds are from Shh+ epithelial cells of tongue primordium in distinction from mature taste bud cells which arise from surrounding tissue compartments.Biochem Biophys Res Commun. 2019 Jul 12;515(1):149-155. doi: 10.1016/j.bbrc.2019.05.132. Epub 2019 May 24. Biochem Biophys Res Commun. 2019. PMID: 31133375 Free PMC article.
-
Anterior and Posterior Tongue Regions and Taste Papillae: Distinct Roles and Regulatory Mechanisms with an Emphasis on Hedgehog Signaling and Antagonism.Int J Mol Sci. 2023 Mar 2;24(5):4833. doi: 10.3390/ijms24054833. Int J Mol Sci. 2023. PMID: 36902260 Free PMC article. Review.
-
Building sensory receptors on the tongue.J Neurocytol. 2004 Dec;33(6):631-46. doi: 10.1007/s11068-005-3332-0. Epub 2005 Oct 11. J Neurocytol. 2004. PMID: 16217619 Review.
Cited by
-
Intrinsic Networks Regulating Tissue Repair: Comparative Studies of Oral and Skin Wound Healing.Cold Spring Harb Perspect Biol. 2022 Nov 1;14(11):a041244. doi: 10.1101/cshperspect.a041244. Cold Spring Harb Perspect Biol. 2022. PMID: 36041785 Free PMC article. Review.
-
High Sox2 expression predicts taste lineage competency of lingual progenitors in vitro.Development. 2023 Feb 15;150(4):dev201375. doi: 10.1242/dev.201375. Epub 2023 Feb 16. Development. 2023. PMID: 36794954 Free PMC article.
-
Gustatory-neuron-supplied R-spondin-2 is required for taste bud replenishment.Stem Cell Reports. 2025 Jul 8;20(7):102542. doi: 10.1016/j.stemcr.2025.102542. Epub 2025 Jun 19. Stem Cell Reports. 2025. PMID: 40541175 Free PMC article.
-
Onset of taste bud cell renewal starts at birth and coincides with a shift in SHH function.Elife. 2021 May 19;10:e64013. doi: 10.7554/eLife.64013. Elife. 2021. PMID: 34009125 Free PMC article.
-
A Scarless Healing Tale: Comparing Homeostasis and Wound Healing of Oral Mucosa With Skin and Oesophagus.Front Cell Dev Biol. 2021 Jul 26;9:682143. doi: 10.3389/fcell.2021.682143. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34381771 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

