Revisiting peptidoglycan sensing: interactions with host immunity and beyond
- PMID: 33057506
- PMCID: PMC7642115
- DOI: 10.1039/d0cc02605k
Revisiting peptidoglycan sensing: interactions with host immunity and beyond
Abstract
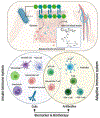
The interaction between host immunity and bacterial cells plays a pivotal role in a variety of human diseases. The bacterial cell wall component peptidoglycan (PG) is known to stimulate an immune response, which makes PG a distinctive recognition element for unveiling these complicated molecular interactions. Pattern recognition receptor (PRR) proteins are among the critical components of this system that initially recognize molecular patterns associated with microorganisms such as bacteria and fungi. These molecular patterns are mostly embedded in the bacterial or fungal cell wall structure and can be released and presented to the immune system in various situations. Nonetheless, detailed knowledge of this recognition is limited due to the diversity among the PG polymer and its fragments; the subsequent responses by multiple hosts add more complexity. Here, we discuss how our understanding of the role and molecular mechanisms of the well-studied PRR, the NOD-like receptors (NLRs), in the human immune system has evolved in recent years. We highlight the instances of other classes of proteins with similar behavior in the recognition of PG that have been identified in other microorganisms such as yeasts. These proteins are particularly interesting because a network of cellular interactions exists between human host cells, bacteria and yeast as a part of the normal human flora. To support our understanding of these interactions, we provide insight into the chemist's toolbox of peptidoglycan probes that aid in the investigations of the behaviors of these proteins and other biological contexts relevant to the sensing and recognition of peptidoglycan. The importance of these interactions in human health for the development of biomarkers and biotherapy is highlighted.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures
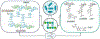

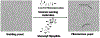

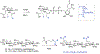

References
-
- Janeway CA and Medzhitov R, Annu. Rev. Immunol, 2002, 20, 197–216. - PubMed
-
- Vollmer W, Blanot D and De Pedro MA, FEMS Microbiol. Rev, 2008, 32, 149–167. - PubMed
-
- Rogers HJ, Ann. N. Y. Acad. Sci, 1974, 235, 29–51. - PubMed
-
- Porfírio S, Carlson RW and Azadi P, Trends Microbiol., 2019, 27, 653–654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

