Early Trastuzumab Interruption and Recurrence-Free Survival in ERBB2-Positive Breast Cancer
- PMID: 33057570
- PMCID: PMC7563661
- DOI: 10.1001/jamaoncol.2020.4749
Early Trastuzumab Interruption and Recurrence-Free Survival in ERBB2-Positive Breast Cancer
Abstract
This cohort study evaluates the association of early trastuzumab interruption with clinical outcomes in patients with ERBB2-positive breast cancer.
Conflict of interest statement
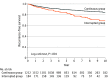
Figures
References
-
- Earl HM, Hiller L, Vallier AL, et al. ; PERSEPHONE Steering Committee and Trial Investigators . 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet. 2019;393(10191):2599-2612. doi:10.1016/S0140-6736(19)30650-6 - DOI - PMC - PubMed
-
- Pivot X, Romieu G, Debled M, et al. ; PHARE trial investigators . 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet. 2019;393(10191):2591-2598. doi:10.1016/S0140-6736(19)30653-1 - DOI - PubMed
-
- Lynce F, Barac A, Geng X, et al. . Prospective evaluation of the cardiac safety of HER2-targeted therapies in patients with HER2-positive breast cancer and compromised heart function: the SAFE-HEaRt study. Breast Cancer Res Treat. 2019;175(3):595-603. doi:10.1007/s10549-019-05191-2 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

