Association of Prehospital Plasma With Survival in Patients With Traumatic Brain Injury: A Secondary Analysis of the PAMPer Cluster Randomized Clinical Trial
- PMID: 33057642
- PMCID: PMC7563075
- DOI: 10.1001/jamanetworkopen.2020.16869
Association of Prehospital Plasma With Survival in Patients With Traumatic Brain Injury: A Secondary Analysis of the PAMPer Cluster Randomized Clinical Trial
Abstract
Importance: Prehospital plasma administration improves survival in injured patients at risk for hemorrhagic shock and transported by air ambulance. Traumatic brain injury (TBI) is a leading cause of death following trauma, but few early interventions improve outcomes.
Objective: To assess the association between prehospital plasma and survival in patients with TBI.
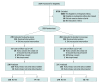
Design, setting, and participants: The Prehospital Air Medical Plasma (PAMPer) trial was a pragmatic, multicenter, phase 3, cluster randomized clinical trial involving injured patients who were at risk for hemorrhagic shock during air medical transport to a trauma center. The trial was conducted at 6 US sites with 9 level-I trauma centers (comprising 27 helicopter emergency services bases). The original trial analyzed 501 patients, including 230 patients who were randomized to receive plasma and 271 randomized to standard care resuscitation. This secondary analysis of a predefined subgroup included patients with TBI. Data analysis was performed from October 2019 to February 2020.
Interventions: Patients were randomized to receive standard care fluid resuscitation or 2 units of thawed plasma.
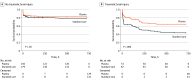
Main outcomes and measures: The primary outcome was mortality at 30 days. Patients with TBI were prespecified as a subgroup for secondary analysis and for measurement of markers of brain injury. The 30-day survival benefit of prehospital plasma in subgroups with and without TBI as diagnosed by computed tomography was characterized using Kaplan-Meier survival analysis and Cox proportional hazard regression.
Results: In total, 166 patients had TBI (median [interquartile range] age, 43.00 [25.00-59.75] years; 125 men [75.3%]). When compared with the 92 patients who received standard care, the 74 patients with TBI who received prehospital plasma had improved 30-day survival even after adjustment for multiple confounders and assessment of the degree of brain injury with clinical variables and biomarkers (hazard ratio [HR], 0.55; 95% CI, 0.33-0.94; P = .03). Receipt of prehospital plasma was associated with improved survival among patients with TBI with a prehospital Glasgow Coma Scale score of less than 8 (HR, 0.56; 95% CI, 0.35-0.91) and those with polytrauma (HR, 0.50; 95% CI, 0.28-0.89). Patients with TBI transported from the scene of injury had improved survival following prehospital plasma administration (HR, 0.45; 95% CI, 0.26-0.80; P = .005), whereas patients who were transferred from an outside hospital showed no difference in survival for the plasma intervention (HR, 1.00; 95% CI, 0.33-3.00; P = .99).
Conclusions and relevance: These findings are exploratory, but they suggest that receipt of prehospital plasma is associated with improved survival in patients with computed tomography-positive TBI. The prehospital setting may be a critical period to intervene in the care of patients with TBI. Future studies are needed to confirm the clinical benefits of early plasma resuscitation following TBI and concomitant polytrauma.
Trial registration: ClinicalTrials.gov Identifier: NCT01818427.
Conflict of interest statement
Figures

Comment in
-
Prehospital Plasma Resuscitation in Patients With Traumatic Brain Injury: PAMPering the Brain.JAMA Netw Open. 2020 Oct 1;3(10):e2017590. doi: 10.1001/jamanetworkopen.2020.17590. JAMA Netw Open. 2020. PMID: 33057639 No abstract available.
References
-
- Galvagno SM Jr, Fox EE, Appana SN, et al. ; PROPPR Study Group . Outcomes after concomitant traumatic brain injury and hemorrhagic shock: a secondary analysis from the Pragmatic, Randomized Optimal Platelets and Plasma Ratios trial. J Trauma Acute Care Surg. 2017;83(4):668-674. doi:10.1097/TA.0000000000001584 - DOI - PMC - PubMed

