Clinical Phenotype and Course of PDE6A-Associated Retinitis Pigmentosa Disease, Characterized in Preparation for a Gene Supplementation Trial
- PMID: 33057649
- PMCID: PMC7563671
- DOI: 10.1001/jamaophthalmol.2020.4206
Clinical Phenotype and Course of PDE6A-Associated Retinitis Pigmentosa Disease, Characterized in Preparation for a Gene Supplementation Trial
Abstract
Importance: Treatment trials require sound knowledge on the natural course of disease.
Objective: To assess clinical features, genetic findings, and genotype-phenotype correlations in patients with retinitis pigmentosa (RP) associated with biallelic sequence variations in the PDE6A gene in preparation for a gene supplementation trial.
Design, setting, and participants: This prospective, longitudinal, observational cohort study was conducted from January 2001 to December 2019 in a single center (Centre for Ophthalmology of the University of Tübingen, Germany) with patients recruited multinationally from 12 collaborating European tertiary referral centers. Patients with retinitis pigmentosa, sequence variants in PDE6A, and the ability to provide informed consent were included.
Exposures: Comprehensive ophthalmological examinations; validation of compound heterozygosity and biallelism by familial segregation analysis, allelic cloning, or assessment of next-generation sequencing-read data, where possible.
Main outcomes and measures: Genetic findings and clinical features describing the entire cohort and comparing patients harboring the 2 most common disease-causing variants in a homozygous state (c.304C>A;p.(R102S) and c.998 + 1G>A;p.?).
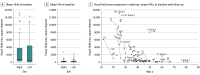
Results: Fifty-seven patients (32 female patients [56%]; mean [SD], 40 [14] years) from 44 families were included. All patients completed the study. Thirty patients were homozygous for disease-causing alleles. Twenty-seven patients were heterozygous for 2 different PDE6A variants each. The most frequently observed alleles were c.304C>A;p.(R102S), c.998 + 1G>A;p.?, and c.2053G>A;p.(V685M). The mean (SD) best-corrected visual acuity was 0.43 (0.48) logMAR (Snellen equivalent, 20/50). The median visual field area with object III4e was 660 square degrees (5th and 95th percentiles, 76 and 11 019 square degrees; 25th and 75th percentiles, 255 and 3923 square degrees). Dark-adapted and light-adapted full-field electroretinography showed no responses in 88 of 108 eyes (81.5%). Sixty-nine of 108 eyes (62.9%) showed additional findings on optical coherence tomography imaging (eg, cystoid macular edema or macular atrophy). The variant c.998 + 1G>A;p.? led to a more severe phenotype when compared with the variant c.304C>A;p.(R102S).
Conclusions and relevance: Seventeen of the PDE6A variants found in these patients appeared to be novel. Regarding the clinical findings, disease was highly symmetrical between the right and left eyes and visual impairment was mild or moderate in 90% of patients, providing a window of opportunity for gene therapy.
Conflict of interest statement
Figures

Comment in
-
Moving Towards PDE6A Gene Supplementation Therapy.JAMA Ophthalmol. 2020 Dec 1;138(12):1251-1252. doi: 10.1001/jamaophthalmol.2020.4216. JAMA Ophthalmol. 2020. PMID: 33057571 Free PMC article. No abstract available.
References
-
- Retinal Information Network Table of contents. https://sph.uth.edu/retnet. Updated 2020. Accessed September 14, 2020.
-
- Dryja TP, Rucinski DE, Chen SH, Berson EL. Frequency of mutations in the gene encoding the alpha subunit of rod cGMP-phosphodiesterase in autosomal recessive retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1999;40(8):1859-1865. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

