A Pharmacokinetic Bridging Study to Compare Systemic Exposure to Budesonide between Budesonide Oral Suspension and ENTOCORT EC in Healthy Individuals
- PMID: 33057953
- PMCID: PMC7691405
- DOI: 10.1007/s40268-020-00324-1
A Pharmacokinetic Bridging Study to Compare Systemic Exposure to Budesonide between Budesonide Oral Suspension and ENTOCORT EC in Healthy Individuals
Abstract
Background and objectives: Currently, there are no US FDA-approved therapies for eosinophilic esophagitis (EoE). Budesonide oral suspension (BOS; SHP621, TAK-721) is a viscous, muco-adherent, oral formulation of budesonide that is in phase III development for the treatment of EoE. BOS 2 mg twice daily was studied in 12- and 36-week phase III studies for the induction and maintenance of clinical remission in adults and adolescents with EoE (NCT02605837 and NCT02736409). ENTOCORT EC is a gelatin capsule formulation of budesonide that is FDA-approved for the treatment of mild-to-moderate active Crohn's disease (CD) in adults and children. This study compared the systemic exposure to budesonide from BOS with that from ENTOCORT EC, aiming to provide the pharmacokinetic (PK) bridge to the safety data of ENTOCORT EC.
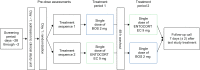
Methods: Healthy adult volunteers (n = 22) were enrolled in an open-label, single-center, crossover study. Participants received a single oral dose of BOS 2 mg and a single oral dose of ENTOCORT EC 9 mg under fasting conditions in a randomized sequence, with a 48-h washout period between treatments. PK parameters were calculated by non-compartmental analysis and compared between treatments using a mixed-effects model with sequence and treatment as fixed effects and individuals within sequence as a random effect.
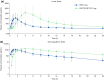
Results: Plasma budesonide concentrations showed that budesonide was absorbed significantly faster from BOS 2 mg than from ENTOCORT EC 9 mg, with peak concentrations reached at 1.5 and 4 h, respectively (p < 0.001). Systemic exposure to budesonide after a single oral dose of BOS 2 mg was lower than that observed after a single oral dose of ENTOCORT EC 9 mg; the least squares geometric mean maximum plasma concentration and the area under the concentration-time curve from the time of dosing to infinity and from the time of dosing to the last measurable concentration of budesonide after BOS 2 mg were 71.1%, 33.5%, and 33.6% of those after ENTOCORT EC 9 mg, respectively. No notable differences in treatment-emergent adverse events were observed between individuals treated with either drug; all events were mild and none resulted in discontinuation from the study.
Conclusions: This study demonstrated that systemic exposure to budesonide after a single oral dose of BOS 2 mg was lower than that after a single oral dose of ENTOCORT EC 9 mg. These results provide PK bridging data to compare BOS with therapeutic doses of ENTOCORT EC with respect to safety information.
Conflict of interest statement
Ivy H. Song, Richard D. Finkelman, and Lan Lan are full-time employees of Shire, a member of the Takeda group of companies, and stockholders of Takeda.
Figures
References
-
- Nonevski IT, Downs-Kelly E, Falk GW. Eosinophilic esophagitis: an increasingly recognized cause of dysphagia, food impaction, and refractory heartburn. Cleve Clin J Med. 2008;75(623–6):9–33. - PubMed
-
- Mukkada V, Falk GW, Eichinger CS, King D, Todorova L, Shaheen NJ. Health-related quality of life and costs associated with eosinophilic esophagitis: a systematic review. Clin Gastroenterol Hepatol. 2018;16(495–503):e8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

