The Pharmacogenetics of Rituximab: Potential Implications for Anti-CD20 Therapies in Multiple Sclerosis
- PMID: 33058021
- PMCID: PMC7851267
- DOI: 10.1007/s13311-020-00950-2
The Pharmacogenetics of Rituximab: Potential Implications for Anti-CD20 Therapies in Multiple Sclerosis
Abstract
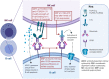
There are a broad range of disease-modifying therapies (DMTs) available in relapsing-remitting multiple sclerosis (RRMS), but limited biomarkers exist to personalise DMT choice. All DMTs, including monoclonal antibodies such as rituximab and ocrelizumab, are effective in preventing relapses and preserving neurological function in MS. However, each agent harbours its own risk of therapeutic failure or adverse events. Pharmacogenetics, the study of the effects of genetic variation on therapeutic response or adverse events, could improve the precision of DMT selection. Pharmacogenetic studies of rituximab in MS patients are lacking, but pharmacogenetic markers in other rituximab-treated autoimmune conditions have been identified. This review will outline the wider implications of pharmacogenetics and the mechanisms of anti-CD20 agents in MS. We explore the non-MS rituximab literature to characterise pharmacogenetic variants that could be of prognostic relevance in those receiving rituximab, ocrelizumab or other monoclonal antibodies for MS.
Keywords: Multiple sclerosis; monoclonal antibody; ocrelizumab; pharmacogenetics; pharmacogenomics; rituximab.
Figures
References
-
- Jokubaitis VG, Spelman T, Kalincik T, et al. Predictors of long-term disability accrual in relapse-onset multiple sclerosis: Predictors of MS Outcomes. Annals of Neurology. 2016;80:89–100. - PubMed
-
- Kalincik T, Manouchehrinia A, Sobisek L, et al. Towards personalized therapy for multiple sclerosis: prediction of individual treatment response. Brain. 2017;140:2426–2443. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources