Early versus delayed continuous positive airway pressure (CPAP) for respiratory distress in preterm infants
- PMID: 33058139
- PMCID: PMC8094884
- DOI: 10.1002/14651858.CD002975.pub2
Early versus delayed continuous positive airway pressure (CPAP) for respiratory distress in preterm infants
Abstract
Background: The application of continuous positive airway pressure (CPAP) has been shown to have some benefits in the treatment of preterm infants with respiratory distress. CPAP has the potential to reduce lung damage, particularly if applied early before atelectasis has occurred. Early application may better conserve an infant's own surfactant stores and consequently may be more effective than later application.
Objectives: • To determine if early compared with delayed initiation of CPAP results in lower mortality and reduced need for intermittent positive-pressure ventilation in preterm infants in respiratory distress ○ Subgroup analyses were planned a priori on the basis of weight (with subdivisions at 1000 grams and 1500 grams), gestation (with subdivisions at 28 and 32 weeks), and according to whether surfactant was used ▫ Sensitivity analyses based on trial quality were also planned ○ For this update, we have excluded trials using continuous negative pressure SEARCH METHODS: We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 6), in the Cochrane Library; Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations Daily and Versions(R); and the Cumulative Index to Nursing and Allied Health Literatue (CINAHL), on 30 June 2020. We also searched clinical trials databases and the reference lists of retrieved articles for randomised controlled trials (RCTs) and quasi-RCTs.
Selection criteria: We included trials that used random or quasi-random allocation to either early or delayed CPAP for spontaneously breathing preterm infants in respiratory distress.
Data collection and analysis: We used the standard methods of Cochrane and Cochrane Neonatal, including independent assessment of trial quality and extraction of data by two review authors. We used the GRADE approach to assess the certainty of evidence.
Main results: We found four studies that recruited a total of 119 infants. Two were quasi-randomised, and the other two did not provide details on the method of randomisation or allocation used. None of these studies used blinding of the intervention or the outcome assessor. Evidence showed uncertainty about whether early CPAP has an effect on subsequent use of intermittent positive-pressure ventilation (IPPV) (typical risk ratio (RR) 0.77, 95% confidence interval (CI) 0.43 to 1.38; typical risk difference (RD) -0.08, 95% CI -0.23 to 0.08; I² = 0%, 4 studies, 119 infants; very low-certainty evidence) or mortality (typical RR 0.93, 95% CI 0.43 to 2.03; typical RD -0.02, 95% CI -0.15 to 0.12; I² = 33%, 4 studies, 119 infants; very low-certainty evidence). The outcome 'failed treatment' was not reported in any of these studies. There was an uncertain effect on air leak (pneumothorax) (typical RR 1.09, 95% CI 0.39 to 3.04, I² = 0%, 3 studies, 98 infants; very low-certainty evidence). No trials reported intraventricular haemorrhage or necrotising enterocolitis. No cases of retinopathy of prematurity were reported in one study (21 infants). One case of bronchopulmonary dysplasia was reported in each group in one study involving 29 infants. Long-term outcomes were not reported.
Authors' conclusions: All four small trials included in this review were performed in the 1970s or the early 1980s, and we are very uncertain whether early application of CPAP confers clinical benefit in the treatment of respiratory distress, or whether it is associated with any adverse effects. Further trials should be directed towards establishing the appropriate level of CPAP and the timing and method of administration of surfactant when used along with CPAP.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
JJH has no interest to declare.
PS has no interest to declare.
AS has no interest to declare.
PGD's institution has a grant pending from the Australian National Health and Medical Research Council (government salary and project support).
Figures
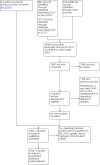

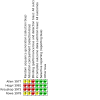










Update of
-
Early versus delayed initiation of continuous distending pressure for respiratory distress syndrome in preterm infants.Cochrane Database Syst Rev. 2002;2002(2):CD002975. doi: 10.1002/14651858.CD002975. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2020 Oct 15;10:CD002975. doi: 10.1002/14651858.CD002975.pub2. PMID: 12076463 Free PMC article. Updated.
References
References to studies included in this review
Allen 1977 {published data only}
Hegyi 1981 {published data only}
Krouskop 1975 {published data only}
Rowe 1978 {unpublished data only}
-
- Rowe JC, Guthrie RD, Hinkes P, Prueitt J, Murphy J, Woodrum DZ, et al. 1017 Time of initiation of CPAP in HMD (Abstract ). Pediatric Research 1978;12:533. [DOI: 10.1203/00006450-197804001-01023] - DOI
References to studies excluded from this review
Badiee 2013 {published data only}
-
- Badiee Z, Naseri F, Sadeghnia A. Early versus delayed initiation of nasal continuous positive airway pressure for treatment of respiratory distress syndrome in premature newborns: a randomized clinical trial. Advanced Biomedical Research 2013;2:4. [DOI: 10.4103/2277-9175.107965] [PMID: ] - DOI - PMC - PubMed
Dunn 2011 {published data only}
Finer 2010 {published data only}
-
- SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network, Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Early CPAP versus surfactant in extremely preterm infants. New England Journal of Medicine 2010;362(21):1970-9. [DOI: 10.1056/NEJMoa0911783] [PMID: ] - DOI - PMC - PubMed
Gerard 1975 {published data only}
John 1976 {published data only}
Mockrin 1975 {published data only}
Morley 2008 {published data only}
Rojas 2009 {published data only}
-
- Rojas MA, Lozano JM, Rojas MX, Laughon M, Bose CL, Rondon MA, et al. Very early surfactant without mandatory ventilation of premature infants treated with early continuous positive airway pressure: a randomized, controlled trial. Pediatrics 2009;123(1):137-42. [DOI: 10.1542/peds.2007-3501] [PMID: ] - DOI - PubMed
Sandri 2010 {published data only}
Tapia 2012 {published data only}
Additional references
Avery 1959
Bahadue 2012
-
- Bahadue FL, Soll R Cochrane Database of Systematic Reviews Art No: CD001456. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD001456. [DOI: 10.1002/14651858.CD001456.pub2] - DOI - PMC - PubMed
Bancalari 1992
-
- Bancalari E, Sinclair JC. Mechanical ventilation. In: Sinclair JC, Bracken MB, editors(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992:200-20.
Barter 1960
Bell 1978
Cogswell 1975
Cotton 1980
Cotton 1998
-
- Cotton RB. Pathophysiology of hyaline membrane disease (excluding surfactant). In: Polin RA, Fox WW, editors(s). Fetal and Neonatal Physiology. 2nd edition. Philadelphia: WB Saunders, 1998:1165-74.
Davis 2003
Evans 1991
Field 1985
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version 3rd September 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Gregory 1971
Gribetz 1959
Harris 1996
-
- Harris TR, Wood ER. Physiologic principles. In: Goldsmith JP, Karotkin EH, editors(s). Assisted Ventilation. 3rd edition. Philadelphia: WB Saunders, 1996:21-68.
Henderson‐Smart 2004
-
- Henderson‐Smart DJ. Recurrent apnoea. In: Evidence Based Pediatrics. Oxford: Blackwell, 2004.
Herman 1973
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA; on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group [Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011]. Available from handbook.cochrane.org.
Higgins 2017
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook.
Ho 2015
Langston 1984
-
- Langston C, Kida K, Reed M, Thurlbeck WM. Human lung growth in late gestation and in the neonate. American Review of Respiratory Disease 1984;129(4):607-13. [PMID: ] - PubMed
Miller 1990
Narendran 2003
Review Manager 2020 [Computer program]
-
- The Cochrane Collaboration Review Manager (RevMan). Version 5.4. The Cochrane Collaboration, 2020.
Richardson 1978
Rojas‐Reyes 2012
Saunders 1976
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Subramaniam 2016
Verder 1994
References to other published versions of this review
Ho 2002
Ho 2007
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

