Continuous positive airway pressure (CPAP) for respiratory distress in preterm infants
- PMID: 33058208
- PMCID: PMC8094155
- DOI: 10.1002/14651858.CD002271.pub3
Continuous positive airway pressure (CPAP) for respiratory distress in preterm infants
Abstract
Background: Respiratory distress, particularly respiratory distress syndrome (RDS), is the single most important cause of morbidity and mortality in preterm infants. In infants with progressive respiratory insufficiency, intermittent positive pressure ventilation (IPPV) with surfactant has been the usual treatment, but it is invasive, potentially resulting in airway and lung injury. Continuous positive airway pressure (CPAP) has been used for the prevention and treatment of respiratory distress, as well as for the prevention of apnoea, and in weaning from IPPV. Its use in the treatment of RDS might reduce the need for IPPV and its sequelae.
Objectives: To determine the effect of continuous distending pressure in the form of CPAP on the need for IPPV and associated morbidity in spontaneously breathing preterm infants with respiratory distress.
Search methods: We used the standard strategy of Cochrane Neonatal to search CENTRAL (2020, Issue 6); Ovid MEDLINE and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Daily and Versions; and CINAHL on 30 June 2020. We also searched clinical trials databases and the reference lists of retrieved articles for randomised controlled trials and quasi-randomised trials.
Selection criteria: All randomised or quasi-randomised trials of preterm infants with respiratory distress were eligible. Interventions were CPAP by mask, nasal prong, nasopharyngeal tube or endotracheal tube, compared with spontaneous breathing with supplemental oxygen as necessary.
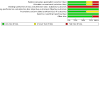
Data collection and analysis: We used standard methods of Cochrane and its Neonatal Review Group, including independent assessment of risk of bias and extraction of data by two review authors. We used the GRADE approach to assess the certainty of evidence. Subgroup analyses were planned on the basis of birth weight (greater than or less than 1000 g or 1500 g), gestational age (groups divided at about 28 weeks and 32 weeks), timing of application (early versus late in the course of respiratory distress), pressure applied (high versus low) and trial setting (tertiary compared with non-tertiary hospitals; high income compared with low income) MAIN RESULTS: We included five studies involving 322 infants; two studies used face mask CPAP, two studies used nasal CPAP and one study used endotracheal CPAP and continuing negative pressure for a small number of less ill babies. For this update, we included one new trial. CPAP was associated with lower risk of treatment failure (death or use of assisted ventilation) (typical risk ratio (RR) 0.64, 95% confidence interval (CI) 0.50 to 0.82; typical risk difference (RD) -0.19, 95% CI -0.28 to -0.09; number needed to treat for an additional beneficial outcome (NNTB) 6, 95% CI 4 to 11; I2 = 50%; 5 studies, 322 infants; very low-certainty evidence), lower use of ventilatory assistance (typical RR 0.72, 95% CI 0.54 to 0.96; typical RD -0.13, 95% CI -0.25 to -0.02; NNTB 8, 95% CI 4 to 50; I2 = 55%; very low-certainty evidence) and lower overall mortality (typical RR 0.53, 95% CI 0.34 to 0.83; typical RD -0.11, 95% CI -0.18 to -0.04; NNTB 9, 95% CI 2 to 13; I2 = 0%; 5 studies, 322 infants; moderate-certainty evidence). CPAP was associated with increased risk of pneumothorax (typical RR 2.48, 95% CI 1.16 to 5.30; typical RD 0.09, 95% CI 0.02 to 0.16; number needed to treat for an additional harmful outcome (NNTH) 11, 95% CI 7 to 50; I2 = 0%; 4 studies, 274 infants; low-certainty evidence). There was no evidence of a difference in bronchopulmonary dysplasia, defined as oxygen dependency at 28 days (RR 1.04, 95% CI 0.35 to 3.13; I2 = 0%; 2 studies, 209 infants; very low-certainty evidence). The trials did not report use of surfactant, intraventricular haemorrhage, retinopathy of prematurity, necrotising enterocolitis and neurodevelopment outcomes in childhood.
Authors' conclusions: In preterm infants with respiratory distress, the application of CPAP is associated with reduced respiratory failure, use of mechanical ventilation and mortality and an increased rate of pneumothorax compared to spontaneous breathing with supplemental oxygen as necessary. Three out of five of these trials were conducted in the 1970s. Therefore, the applicability of these results to current practice is unclear. Further studies in resource-poor settings should be considered and research to determine the most appropriate pressure level needs to be considered.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
JJH: none.
PS: none.
PGD's institution has a grant pending from the Australian National Health and Medical Research Council.
Figures
















Update of
-
Continuous distending pressure for respiratory distress in preterm infants.Cochrane Database Syst Rev. 2015 Jul 4;2015(7):CD002271. doi: 10.1002/14651858.CD002271.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2020 Oct 15;10:CD002271. doi: 10.1002/14651858.CD002271.pub3. PMID: 26141572 Free PMC article. Updated.
References
References to studies included in this review
Belenky 1976 {published and unpublished data}
-
- Belenky DA, Orr RJ, Woodrum DE, Hodson WA. Is continuous transpulmonary pressure better than conventional respiratory management of hyaline membrane disease? A controlled study. Pediatrics 1976;58(6):800-8. - PubMed
Buckmaster 2007 {published and unpublished data}
-
- Buckmaster AG, Gaston A, Wright IM, Foster JP, Henderson-Smart DJ. Continuous positive airway pressure therapy for infants with respiratory distress in non tertiary care centers: a randomized controlled trial. Pediatrics 2007;120(3):509-18. - PubMed
Durbin 1976 {published data only}
Mwatha 2020 {published data only}
Rhodes 1973 {published data only}
-
- Rhodes PG, Hall RT. Continuous positive airway pressure delivered by face mask in infants with the idiopathic respiratory distress syndrome: a controlled study. Pediatrics 1973;52(1):1-5. - PubMed
References to studies excluded from this review
Afjeh 2017 {published data only}
-
- Afjeh SA, Sabzehei MK, Khoshnood Shariati M, Shamshiri AR, Esmaili F. Evaluation of initial respiratory support strategies in VLBW neonates with RDS. Archives of Iranian Medicine 2017;20(3):158-64. [PMID: ] - PubMed
Badiee 2013 {published data only}
Celik 2018 {published data only}
-
- Celik M, Bulbul A, Uslu S, Dursun M, Guran O, Kiray Bas E, et al. A comparison of the effects of invasive mechanic ventilation/surfactant therapy and non-invasive nasal-continuous positive airway pressure in preterm newborns. Journal of Maternal-fetal & Neonatal Medicine 2018;31(24):3225-31. [PMID: ] - PubMed
Colnaghi 2008 {published data only}
-
- Colnaghi N, Matassa PG, Fumagalli M, Messina D, Mosca F. Pharyngeal pressure value using two continuous positive airway pressure devices. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(4):F302-4. - PubMed
Dunn 2011 {published data only}
-
- Dunn MS, Kaempf J, Klerk A, Klerk R, Reilly M, Howard D, et al. Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics 2011;128(5):e1069-76. [PMID: ] - PubMed
Fanaroff 1973 {published data only}
-
- Fanaroff AA, Cha CC, Sosa R, Crumrine RS, Klaus MH. Controlled trial of continuous negative external pressure in the treatment of severe respiratory distress syndrome. Journal of Pediatrics 1973;82(6):921-8. - PubMed
Finer 2010 {published data only}
Heiring 2015 {published data only}
-
- Heiring C, Steensberg J, Bjerager M, Greisen G. A randomized trial of low-flow oxygen versus nasal continuous positive airway pressure in preterm infants. Neonatology 2015;108(4):259-65. [PMID: ] - PubMed
Morley 2008 {published data only}
-
- Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB, for the COIN Trial Investigators. Nasal CPAP or intubation at birth for very preterm infants. New England Journal of Medicine 2008;358(7):700-8. - PubMed
Pieper 2003 {published data only}
-
- Pieper CH, Smith J, Maree D, Pohl FC. Is nCPAP of value in extreme preterms with no access to neonatal intensive care? Journal of Tropical Pediatrics 2003;49(3):148-52. - PubMed
Rojas 2009 {published data only}
-
- Rojas MA, Lozano JM, Rojas MX, Laughon M, Bose CL, Rondon MA, et al. Very early surfactant without mandatory ventilation in premature infants treated with early continuous positive airway pressure: a randomized, controlled trial. Pediatrics 2009;123(1):137-42. - PubMed
Samuels 1996 {published data only}
-
- Samuels MP, Raine J, Wright T, Alexander JA, Lockyer K, Spencer A, et al. Continuous negative extrathoracic pressure in neonatal respiratory failure. Pediatrics 1996;98(6 Pt 1):1154-60. - PubMed
-
- Telford K, Waters L, Vyas H, Manktelow BN, Draper ES, Marlow N. Outcome after neonatal continuous negative-pressure ventilation: follow-up assessment. Lancet 2006;367(9516):1080-5. - PubMed
Sandri 2010 {published data only}
-
- Sandri F, Plavka R, Ancora G, Simeoni U, Stranak Z, Martinelli S, et al. Prophylactic or early selective surfactant combined with nCPAP in very preterm infants. Pediatrics 2010;124(6):e1402-9. - PubMed
Swyer 1973 {published data only}
-
- Swyer PR, Bryan MH, Chance GW, McMurray SB, Olinsky A, Reilly B. Continuous pressure breathing in RDS: comparative trial of 3 methods. INSERM Paris 1973.
Tapia 2012 {published data only}
-
- Tapia JL, Urzua S, Bancalari A, Meritano J, Torres G, Fabres J, et al. Randomized trial of early bubble continuous positive airway pressure for very low birth weight infants. Journal of Pediatrics 2012;161(1):75-80.e1. [PMID: ] - PubMed
Tooley 2003 {published data only}
-
- Tooley J, Dyke M. Randomized study of nasal continuous positive airway pressure in the preterm infant with respiratory distress syndrome. Acta Paediatrica 2003;92(10):1170-4. - PubMed
References to ongoing studies
NCT04209946 {published data only}
-
- NCT04209946. Early caffeine and LISA compared to caffeine and CPAP in preterm infants (CaLI). clinicaltrials.gov/ct2/show/record/NCT04209946 (first received 24 December 2019).
Additional references
Bancalari 1992
-
- Bancalari E, Sinclair JC. Mechanical ventilation. In: Sinclair JC, Bracken MB, editors(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992:200-220.
Cummings 2016
-
- Cummings JJ, Polin RA. AAP the Committee on fetus and newborn. Noninvasive Respiratory Support. Pediatrics 2016;137(1):e20153758. - PubMed
Davis 2003
Deeks 2011
-
- Deeks JJ, Higgins JP, Altman D. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
De Paoli 2008
-
- De Paoli AG, Davis PG, Faber B, Morley CJ. Devices and pressure sources for administration of nasal continuous positive airway pressure (NCPAP) in preterm neonates. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD002977. [DOI: 10.1002/14651858.CD002977.pub2] [CD002977] - DOI - PMC - PubMed
Elgellab 2001
-
- Elgellab A, Riou Y, Abbazine A, Truffert P, Matran R, Lequien P, et al. Effects of nasal continuous positive airway pressure (NCPAP) on breathing pattern in spontaneously breathing premature newborn infants. Intensive Care Medicine 2001;27(11):1782-7. [PMID: ] - PubMed
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Gregory 1971
-
- Gregory GA, Kitterman JA, Phibbs RH, Tooley WH, Hamilton WK. Treatment of the idiopathic respiratory-distress syndrome with continuous positive airway pressure. New England Journal of Medicine 1971;284(24):1333-40. [PMID: ] - PubMed
Higgins 2017
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Ho 2004
Manuck 2016
-
- Manuck TA, Rice MM, Bailit JL, Grobman WA, Reddy UM, Wapner RJ, et al, Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. American Journal of Obstetrics and Gynecology 2016;215(1):103.e1-103.e14. [PMID: ]
Martin 1977
-
- Martin RJ, Nearman HS, Katona PG, Klaus MH. The effect of a low continuous positive airway pressure on the reflex control of respiration in the preterm infant. Journal of Pediatrics 1977;90(6):976-81. [PMID: ] - PubMed
Martin 2014
-
- Martin S, Duke T, Davis P. Efficacy and safety of bubble CPAP in neonatal care in low and middle income countries: a systematic review. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(6):F495-504. [PMID: ] - PubMed
Mukerji 2019
-
- Mukerji A, Wahab MG, Mitra S, Mondal T, Paterson D, Beck J, et al. High continuous positive airway pressure in neonates: a physiological study. Pediatric Pulmonology 2019;7:1039-44. [PMID: ] - PubMed
Narendran 2003
-
- Narendran V, Donovan EF, Hoath SB, Akinbi HT, Steichen JJ, Jobe AH. Early bubble CPAP and outcomes in ELBW preterm infants. Journal of Perinatology 2003;23(3):195-9. - PubMed
NICE 2019
-
- National Institute for Health and Care Excellence. Specialist neonatal respiratory care for babies born preterm, 2019. nice.org.uk/guidance/ng124 (accessed 27 May 2019). - PubMed
Pape 1976
-
- Pape KE, Armstrong DL, Fitzharding PM. Central nervous system pathology associated with mask ventilation in the very low birth weight infant: a new etiology for intracerebellar hemorrhages. Pediatrics 1976;58(4):473-83. - PubMed
Ramaswamy 2020
-
- Ramaswamy VV, More K, Roehr CC, Bandiya P, Nangia S. Efficacy of noninvasive respiratory support modes for primary respiratory support in preterm neonates with respiratory distress syndrome: Systematic review and network meta-analysis. Pediatric Pulmonology 2020. [PMID: ] - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richardson 1978
-
- Richardson CP, Jung AL. Effects of continuous positive airway pressure on pulmonary function and blood gases of infants with respiratory distress syndrome. Pediatric Research 1978;12(7):771-4. [PMID: ] - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s), GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendations. Available from gdt.gradepro.org/app/handbook/handbook.html. Updated October 2013.
Soll 1998
Srikasibhandha 1974
Subramaniam 2016
WHO 2012
-
- World Health Organization. Born too soon: the global action report on preterm birth, 2012. www.who.int/pmnch/media/news/2012/201204_borntoosoon-report.pdf (accessed prior to 25 September 2020).
WHO 2015
-
- World Health Organization. WHO recommendation on continuous positive airway pressure therapy for the treatment of preterm newborns with respiratory distress syndrome. extranet.who.int/rhl/topics/newborn-health/care-newborn-infant/who-recom... (accessed prior to 25 September 2020).
Woodrum 1972
-
- Woodrum DE, Oliver TK Jr, Hodson WA. The effect of prematurity and hyaline membrane disease on oxygen exchange in the lung. Pediatrics 1972;50(3):380-6. [PMID: ] - PubMed
References to other published versions of this review
Ho 2000
Ho 2002
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

